3.6.1 Define enzyme and active site
Enzyme: A globular protein that increases the rate of a biochemical reaction by lowering the activation energy threshold (i.e. a biological catalyst)
Active Site: The site on the surface of an enzyme which binds to the substrate molecule
![]() Activation Energy Threshold
Activation Energy Threshold
3.6.2 Explain enzyme-substrate specificity
Active site and substrate complement each other in terms of both shape and chemical properties (e.g. opposite charges)
Binding to the active site brings the substrate into close physical proximity, creating an enzyme-substrate complex
The enzyme catalyses the conversion of the substrate into a product (or products), creating an enzyme-product complex
As the enzyme is not consumed in the reaction, it can continue to work once the product dissociates (hence only low concentrations are needed)
Enzyme-Substrate Specificity
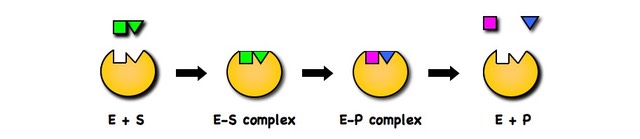
Lock and Key Model
Enzymes and substrates share specificity (a given enzyme will only interact with a small number of specific substrates that complement the active site)
This explanation of enzyme-substrate interaction is described as the 'lock and key' model (a lock only opens in response to a specific key)
![]() Compare with Induced Fit Model (7.6.2)
Compare with Induced Fit Model (7.6.2)
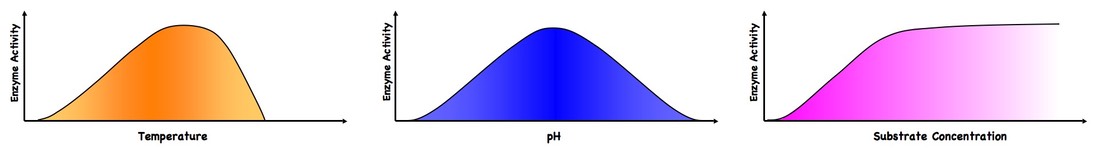
3.6.3 Explain the effects of temperature, pH and substrate concentration on enzyme activity
Temperature
- Low temperatures result in insufficient thermal energy for the activation of a given enzyme-catalysed reaction to be achieved
- Increasing the temperature will increase the speed and motion of both enzyme and substrate, resulting in higher enzyme activity
- This is because a higher kinetic energy will result in more frequent collisions between enzyme and substrate
- At an optimal temperature (may differ for different enzymes), the rate of enzyme activity will be at its peak
- Higher temperatures will cause enzyme stability to decrease, as the thermal energy disrupts the hydrogen bonds holding the enzyme together
- This causes the enzyme (particularly the active site) to lose its shape, resulting in a loss of enzyme activity (denaturation)
pH
- Changing the pH will alter the charge of the enzyme, which in turn will protein solubility and may change the shape of the molecule
- Changing the shape or charge of the active site will diminish its ability to bind to the substrate, abrogating enzyme function
- Enzymes have an optimum pH (may differ between enzymes) and moving outside of this range will always result in a diminished rate of reaction
Substrate Concentration
- Increasing substrate concentration will increase the activity of a particular enzyme
- More substrate means there is an increased likelihood of enzyme and substrate colliding and reacting, such that more reactions will occur and more products will be formed in a given time period
- After a certain point, the rate of reaction will cease to rise regardless of further increases to substrate concentration, as the environment has become saturated with substrate and all enzymes are bound and reacting (Vmax)
Factors Affecting Enzyme Activity
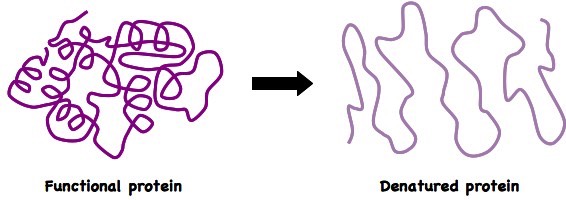
3.6.4 Define denaturation
Denaturation is a structural change in a protein that results in the loss (usually permanent) of its biological properties
- Heat and pH are two agents which may cause denaturation of an enzyme
Denaturation
3.6.5 Explain the use of lactase in the production of lactose-free milk
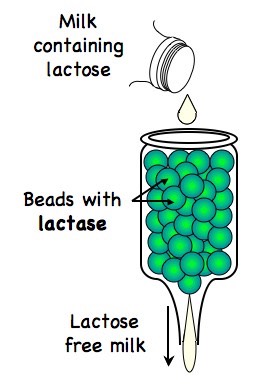
Lactose is a disaccharide of glucose and galactose which can be broken down by the enzyme lactase
Historically, mammals exhibit a marked decrease in lactase production after weaning - leading to lactose intolerance (incidence is particularly high in Asian / African / Native American / Aboriginal populations)
Lactose-free milk can be produced by purifying lactase (e.g. from yeast or bacteria) and binding it to an inert substance (such as alginate beads)
Milk passed over this immobilised enzyme will become lactose-free
The generation of lactose-free milk can be used in a number of ways:
- As a source of milk for lactose-intolerant individuals
- As a means to increase the sweetness of milk (glucose and galactose are sweeter in flavour), thus negating the need for artificial sweeteners
- As a way of reducing the crystallisation of ice-creams (glucose and galactose are more soluble than lactose)
- As a means of shortening the production time for yogurts or cheese (bacteria ferment glucose and galactose more readily than lactose)