7.6.1 State that metabolic pathways consist of chains and cycles of enzyme-catalysed reactions
- Most chemical changes in a cell results from chains and cycles of reactions, with each step controlled by a separate specific enzyme
- This allows for a far greater level of control and regulation of metabolic pathways (such as photosynthesis and cell respiration)
7.6.2 Describe the induced fit model
- When enzymes and substrates bind, the active site is not completely rigid and may undergo a conformational change in shape to better fit the substrate
- This conformational change may increase the reactivity of the substrate and be necessary for the enzyme's catalytic activity
- The induced fit model explains how an enzyme may be able to bind to, and catalyse, several different substrates (broad specificity)
The Induced Fit Model

7.6.3 Explain that enzymes lower the activation energy of the chemical reactions that they catalyse
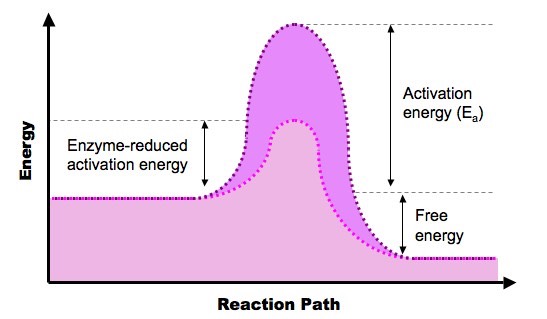
- Every reaction requires a certain amount of energy to proceed - this is the activation energy (Ea)
- Enzymes speed up the rate of a biochemical reaction by lowering the activation energy
- If more energy is in the products than the reactants, energy is lost from the system (endergonic)
- These reactions are usually anabolic (building things up), as the energy is being used up in bond formation between two substrate molecules
- If more energy is in the reactants than the products, excess energy is released into the system (exergonic)
- These reactions are usually catabolic (breaking things down), as the energy is released from the broken bonds within molecules
Reaction Pathway of a Typical Exergonic / Exothermic Reaction
7.6.4 Explain the difference between competitive and non-competitive inhibition, with reference to one example of each
Competitive Inhibition
- A molecule (inhibitor) which is structurally / chemically similar to the substrate and binds to the active site of the enzyme
- This serves to block the active site and thus prevent substrate binding (competes for the active site)
- Its effect can be reduced by increasing substrate concentration
Example: Relenza is a competitive inhibitor of neuraminidase (influenza virus enzyme), preventing the release of virions from infected cells
Non-competitive Inhibition
- A molecule (inhibitor) which is not structurally or chemically similar to the substrate and binds to a site other than the active site (allosteric site)
- This causes a conformational change in the active site, meaning the substrate cannot bind
- Its effect cannot be reduced by increasing substrate concentration as it is not competing for the active site
Example: Cyanide (CN-) inhibits enzymes (cytochrome oxidase) in the electron transport chain by breaking disulphide bonds within the enzyme
Competitive versus Non-competitive Inhibition

7.6.5 Explain the control of metabolic pathways by end-product inhibition, including the role of allosteric sites
End-product inhibition is a form of negative feedback in which increased levels of product decrease the rate of product formation
- Because metabolic pathways usually consist of chains (e.g. glycolysis) or cycles (e.g. Krebs cycle), the product can regulate the rate of its own production by inhibiting an earlier enzyme in the metabolic pathway
- The product binds to an allosteric site of an enzyme, causing a conformational change in the active site (non-competitive inhibition)
- As the enzyme can not currently function, the rate of product formation will decrease (and with less product there is less enzyme inhibition)
End-Product Inhibition
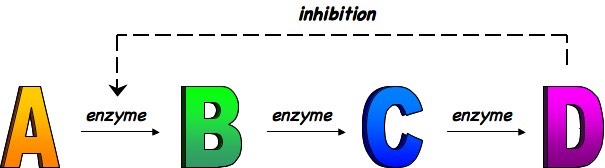
An example of end-product inhibition is the regulation of ATP formation by phosphofructokinase (an enzyme in glycolysis)
- ATP inhibits phosphofructokinase, so that when ATP levels are high, glucose is not broken down (but instead can be stored as glycogen)
- When ATP levels are low, phosphofructokinase is activated and glucose is broken down to make more ATP