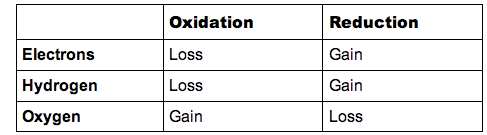
8.1.1 State that oxidation involves the loss of electrons from an element, whereas reduction involves a gain of electrons and that oxidation frequently involves gaining oxygen or losing hydrogen, whereas reduction frequently involves losing oxygen or gaining hydrogen

- Redox (reduction-oxidation) reactions are chemical reactions that involve the transfer of electrons (gain or loss) between species
- Mnemonics for redox reactions include:
- OIL RIG: Oxidation Is Loss (of electrons), Reduction Is Gain (of electrons)
- ELMO: Electron Loss Means Oxidation
- LEO goes GER: Loss of Electrons is Oxidation, Gain of Electrons is Reduction
- In metabolic reactions, a species that has been reduced has the ability to reduce other species (this is the predominant role of hydrogen carriers)
The differences between oxidation and reduction can be summarised by the following table:
8.1.2 Outline the process of glycolysis, including phosphorylation, lysis, oxidation and ATP formation

- Glycolysis is the first stage of cell respiration and involves the breakdown of glucose into two molecules of pyruvate
- It is an anaerobic reaction (does not require the presence of oxygen) and occurs in the cytoplasm
There are four main parts in glycolysis (not including intermediary steps):
1. Phosphorylation: A hexose sugar is phosphorylated by two ATP to become hexose biphosphate
2. Lysis: The hexose biphosphate splits into two triose phosphates (3C sugars)
3. Oxidation: Hydrogen removed from the triose phosphates via oxidation (NAD is reduced to NADH + H+)
4. ATP Formation: Four ATP molecules are released as the triose phosphates are converted into pyruvate
- Overall: One molecule of glucose results in 2 pyruvate, 2 (NADH + H+) and 2 ATP (net gain)
8.1.3 Draw and label a diagram showing the structure of a mitochondrion as seen in electron micrographs
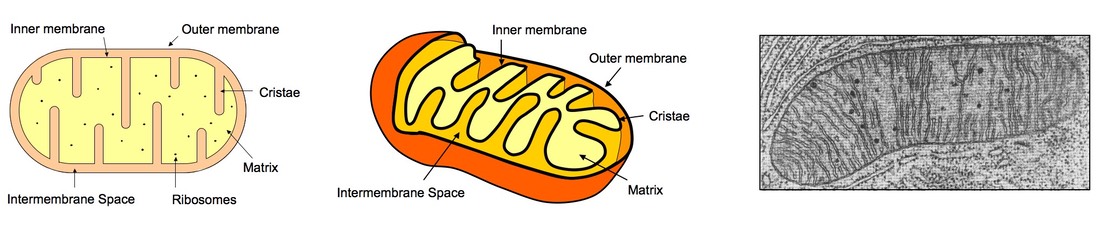
2D Representation 3D Representation Electron Micrograph
8.1.4 Explain aerobic respiration, including the link reaction, the Krebs cycle, the role of NADH + H+, the electron transport chain and the role of oxygen
- Aerobic respiration takes place in the mitochondria, using the pyruvate produced via glycolysis
- It produces large amounts of ATP in the presence of oxygen via three main processes:
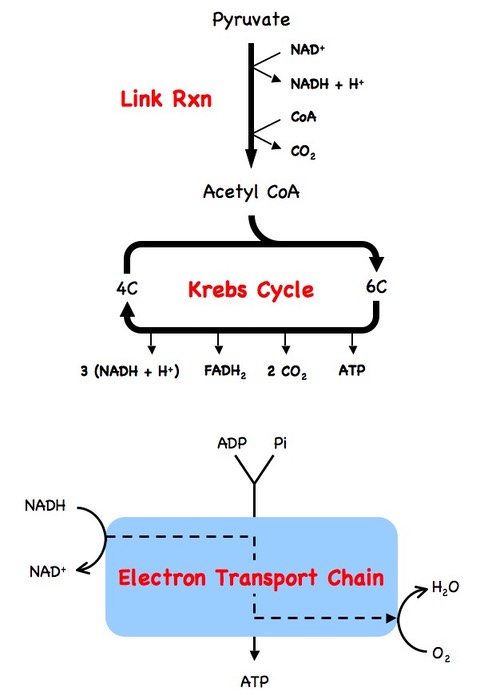
The Link Reaction
- Pyruvate is transported from the cytosol to the mitochondrial matrix in a reaction that produces (one) NADH + H+ via oxidation
- The pyuvate loses a carbon (as CO2) and the remaining two carbons are complexed with coenzyme A (CoA) to form acetyl CoA
The Krebs Cycle
- In the matrix, acetyl CoA combines with a 4C compound to form a 6C compound
- Over a series of reactions the 6C compound is broken back down into the original 4C compound
- These reactions result in the formation of 2 CO2 molecules, 1 ATP molecule and multiple hydrogen carriers, specifically 3 (NADH + H+) and 1 FADH2
The Electron Transport Chain
- The hydrogen carriers (NADH + H+ and FADH2) provide electrons to the electron transport chain on the inner mitochondrial membrane
- As the electrons cycle through the chain they lose energy, which is used to translocate H+ ions to the intermembrane space (creating a gradient)
- The hydrogen ions return to the matrix through the transmembrane enzyme ATP synthase, producing multiple ATP molecules (via chemiosmosis)
- Oxygen acts as a final electron acceptor for the electron transport chain, allowing further electrons to enter the chain
- Oxygen combines the electrons with H+ ions to form water molecules
- The electron transport chain produces the majority of the ATP molecules produced via aerobic respiration (~32 out of 36 ATP molecules)
![]() Overview of Aerobic Respiration
Overview of Aerobic Respiration
8.1.5 Explain oxidative phosphorylation in terms of chemiosmosis
- Oxidative phosphorylation describes the production of ATP from oxidised hydrogen carriers (as opposed to substrate level phosphorylation)
- When electrons are donated to the electron transport chain, they lose energy as they are passed between successive carrier molecules
- This energy is used to translocate H+ ions from the matrix to the intermembrane space against the concentration gradient
- The build up of H+ ions creates an electrochemical gradient, or proton motive force (PMF)
- The protons return to the matrix via a transmembran enzyme called ATP synthase
- As they return they release energy which is used to produce ATP (from ADP and Pi)
- This process is called chemiosmosis and occurs in the cristae
- The H+ ions and electrons are combined with oxygen to form water, allowing the process to be repeated anew
Overview of Chemiosmosis

8.1.6 Explain the relationship between the structure of the mitochondria and its function
- Inner membrane: Folded into cristae to increase surface area for electron transport chain
- Intermembrane space: Small space between inner and outer membranes for accumulation of protons (increases PMF)
- Matrix: Contains appropriate enzymes and a suitable pH for the Krebs cycle to occur
- Outer membrane: Contains appropriate transport proteins for shuttling pyruvate into the mitochondria