Water Structure
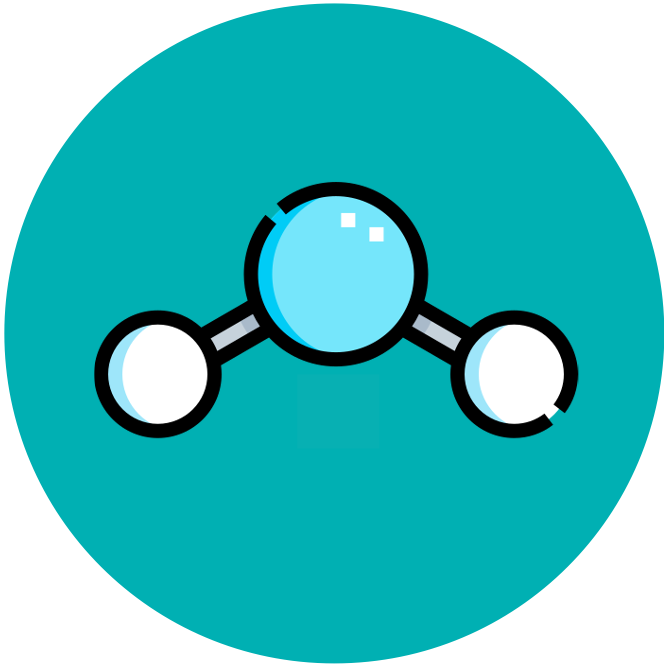
Water is made up of two hydrogen atoms covalently bonded to an oxygen atom (molecular formula = H2O)
-
While this covalent bonding involves the sharing of electrons, they are not shared equally between the atoms, resulting in polarity
Polarity describes the slight difference in charge that occurs at the different poles of the water molecule
-
Oxygen (due to having a higher electronegativity) attracts the electrons more strongly, forming a slightly negative charge (δ–)
-
The hydrogen atoms have a weaker attraction towards the electrons, resulting in a slightly positive charge (δ+)
The polarity of water allows it to form weak associations with other polar molecules or charged ions
-
The slightly negative poles (δ–) will attract the slightly positive poles (δ+) of other molecules, and vice versa
Polarity of Water

Structure of Water