Water
SL Content Statements
-
A1.1.1
Water as the medium for life
-
Students should appreciate that the first cells originated in water and that water remains the medium in which most processes of life occur.
-
A1.1.2
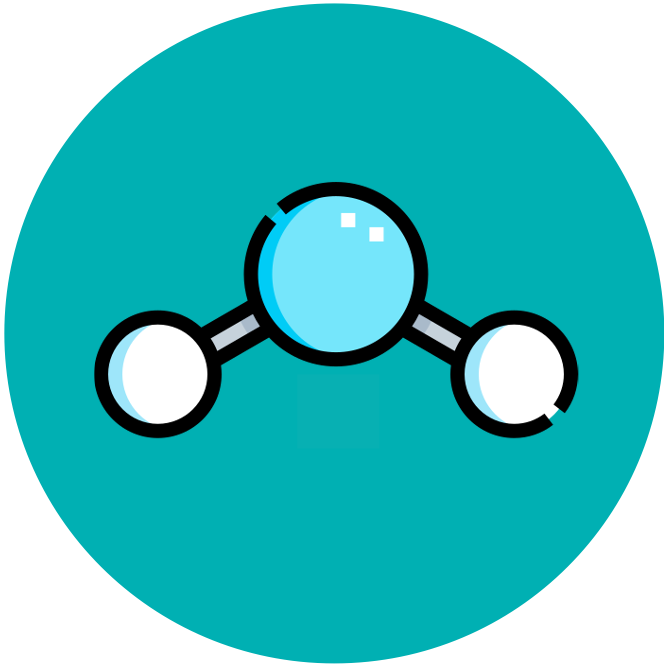
Hydrogen bonds as a consequence of the polar covalent bonds within water molecules
-
Students should understand that polarity of covalent bonding within water molecules is due to unequal sharing of electrons and that hydrogen bonding due to this polarity occurs between water molecules. Students should be able to represent two or more water molecules and hydrogen bonds between them with the notation shown below to indicate polarity.
-
A1.1.3
Cohesion of water molecules due to hydrogen bonding and consequences for organisms
-
Include transport of water under tension in xylem and the use of water surfaces as habitats due to the effect known as surface tension.
-
A1.1.4
Adhesion of water to materials that are polar or charged and impacts for organisms
-
Include capillary action in soil and in plant cell walls.
-
A1.1.5
Solvent properties of water linked to its role as a medium for metabolism and for transport in plants and animals
-
Emphasize that a wide variety of hydrophilic molecules dissolve in water and that most enzymes catalyse reactions in aqueous solution. Students should also understand that the functions of some molecules in cells depend on them being hydrophobic and insoluble.
-
A1.1.6
Physical properties of water and the consequences for animals in aquatic habitats
-
Include buoyancy, viscosity, thermal conductivity and specific heat capacity. Contrast the physical properties of water with those of air and illustrate the consequences using examples of animals that live in water and in air or on land, such as the black-throated loon (Gavia arctica) and the ringed seal (Pusa hispida).
-
D2.3.1
Solvation with water as the solvent
-
Include hydrogen bond formation between solute and water molecules, and attractions between both positively and negatively charged ions and polar water molecules.