Types of Proteins
Proteins can be typically classed as either globular or fibrous according to the organisation of their tertiary structure
-
Other common protein classes include membrane proteins and disordered proteins (lacking a fixed tertiary structure)
Globular Proteins
-
Globular proteins are typically round / spherical in shape and have an irregular amino acid sequence
-
They have functional roles within an organism or cell (they usually carry out a specific biological activity)
-
These proteins are typically soluble in water and are usually more sensitive to changes in temperature and pH
-
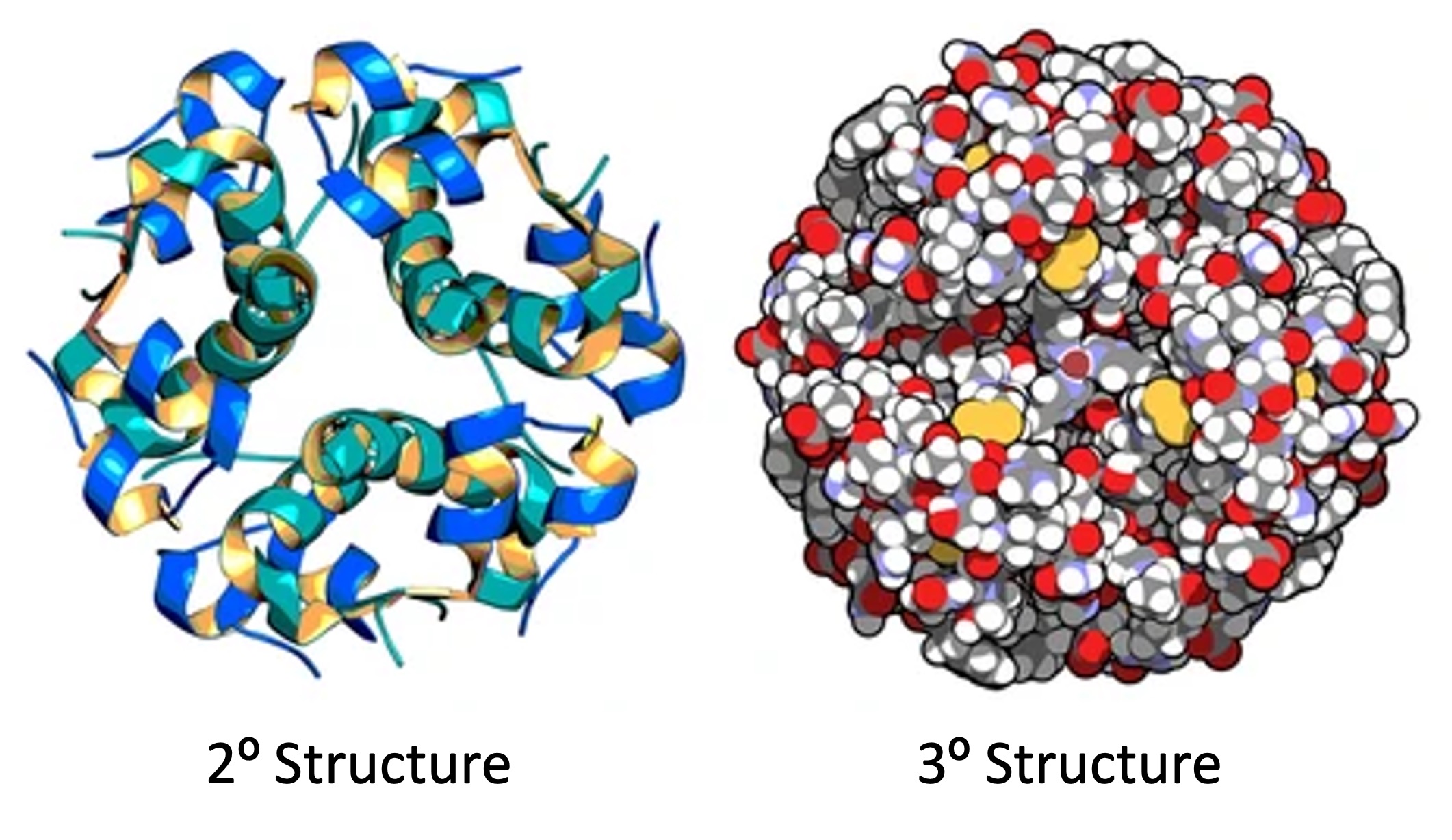
An example of a globular protein is insulin (a protein hormone responsible for blood sugar regulation)
-
Insulin is composed of two polypeptide chains connected by disulphide bridges (between cysteine residues)
-
The two chains associate via non-polar surfaces (hydrophobic) while the exterior of the dimer remains hydrophilic
-
The hydrophilic exterior allows insulin to travel freely within the bloodstream to distant target sites
-
Fibrous Proteins
-
Fibrous proteins are typically long / narrow in shape due to a repetitive amino acid sequence
-
They have structural roles within an organism or cell (help to maintain shape by providing a scaffold)
-
These proteins are typically insoluble in water and are usually less sensitive to changes in temperature and pH
-
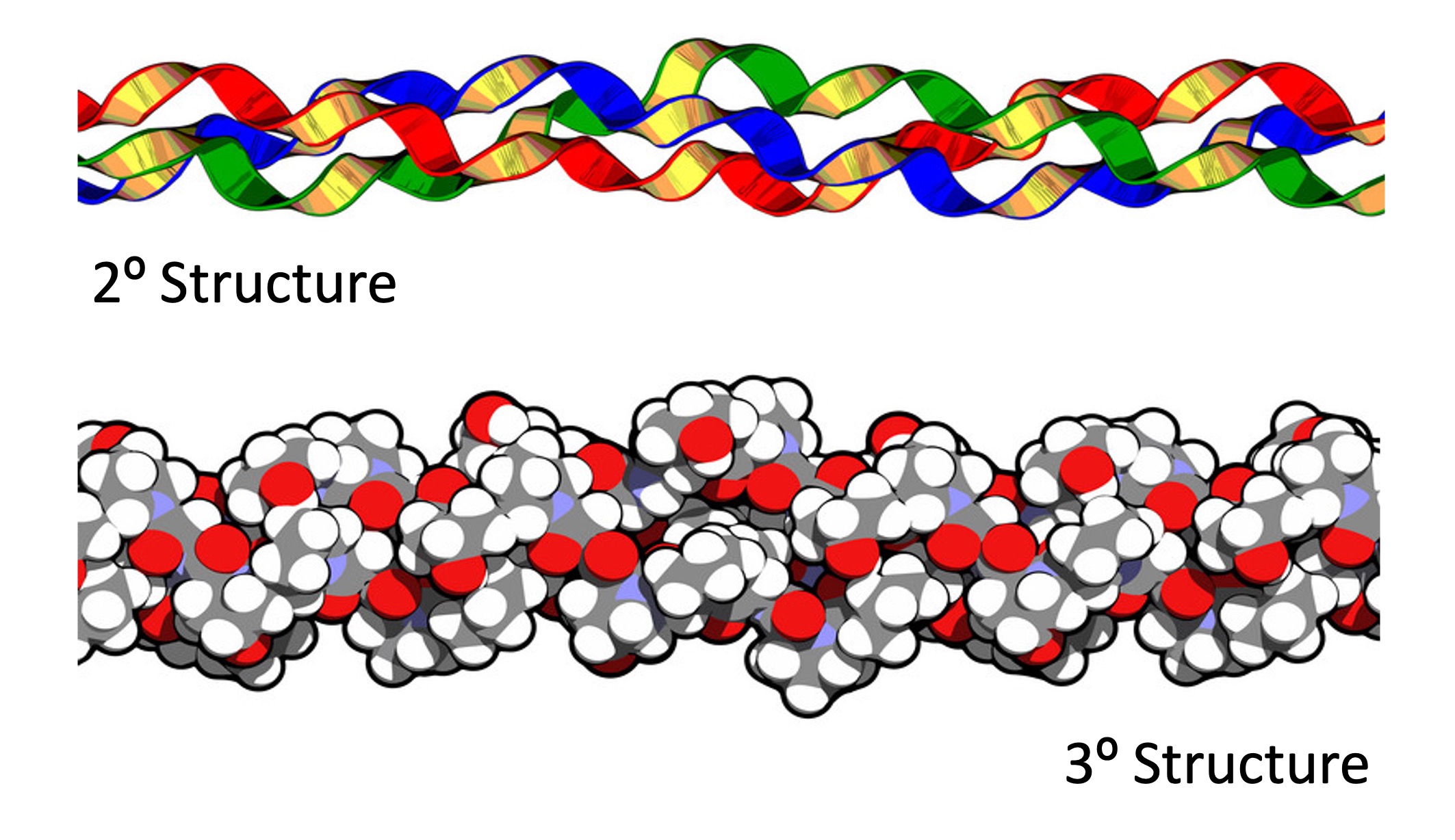
An example of a fibrous protein is collagen (a component of the extracellular matrix ; found in connective tissues)
-
Collagen is composed of amino acid chains bound together to form a triple helix (with covalent cross-linking)
-
It is the most abundant protein in mammals and is found in blood vessels, bones, cartilage, tendons, ligaments and skin
-
The outer surface of the collagen helix is typically non-polar (hydrophobic), allowing collagen to maintain its structure in aqueous solutions
-
Globular vs Fibrous Proteins
Insulin (Globular)
Collagen (Fibrous)
Amino Acid Properties
Amino acids may be either polar (hydrophilic) or non-polar (hydrophobic) depending on the composition of the side chain
-
For example, amine and carboxyl components of R-groups can become positively or negatively charged by binding or dissociating from hydrogen ions
The specific location of polar and non-polar amino acids in a polypeptide sequence will influence the folding and resulting function of a protein
-
Water soluble proteins tend to have non-polar amino acids clustered at their core (for stability) while surface amino acids tend to be polar
-
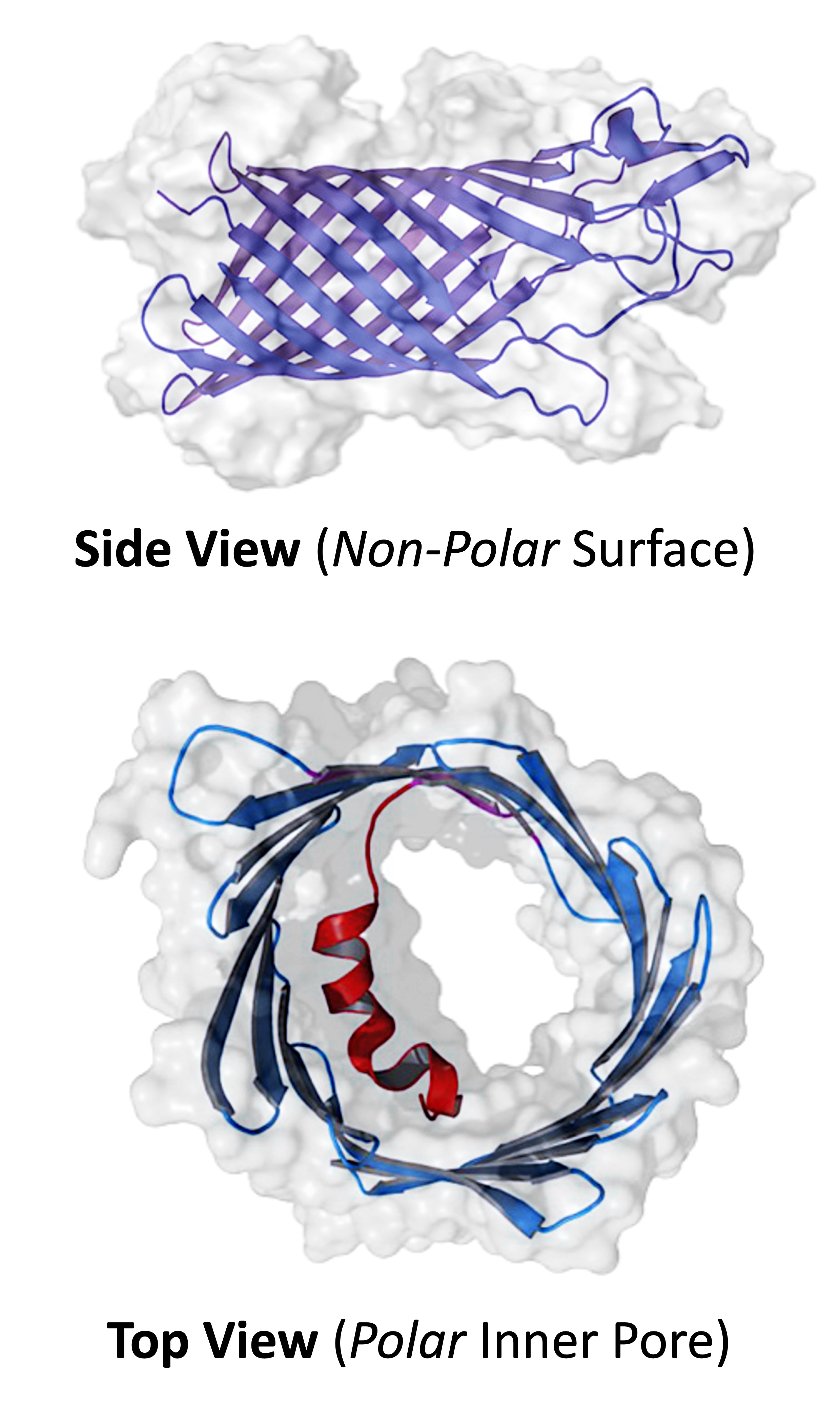
Integral membrane proteins have non-polar amino acids on their surface (to interact with fatty acid tails) while polar amino acids form internal hydrophilic channels / pores
-
Enzyme active sites rely on amino acid polarity in order to associate with a specific substrate (e.g. lipase has a relatively hydrophobic active site)
Membrane Protein Polarity