Simple Diffusion
Diffusion is the net movement of molecules from a region of high concentration to a region of low concentration
-
This directional movement along a gradient is passive and will continue until molecules become evenly dispersed (equilibrium)
The rate of diffusion can be influenced by a number of factors, including:
-
Temperature (affects kinetic energy of particles in solution)
-
Molecular size (larger particles are subjected to greater resistance within a fluid medium)
-
Steepness of gradient (rate of diffusion will be greater with a higher concentration gradient)
Simple Diffusion
Simple diffusion occurs when small and lipophilic molecules pass between phospholipids to freely cross the bilayer
-
Small, non-polar gases (such as oxygen and carbon dioxide) will cross the plasma membrane via simple diffusion
-
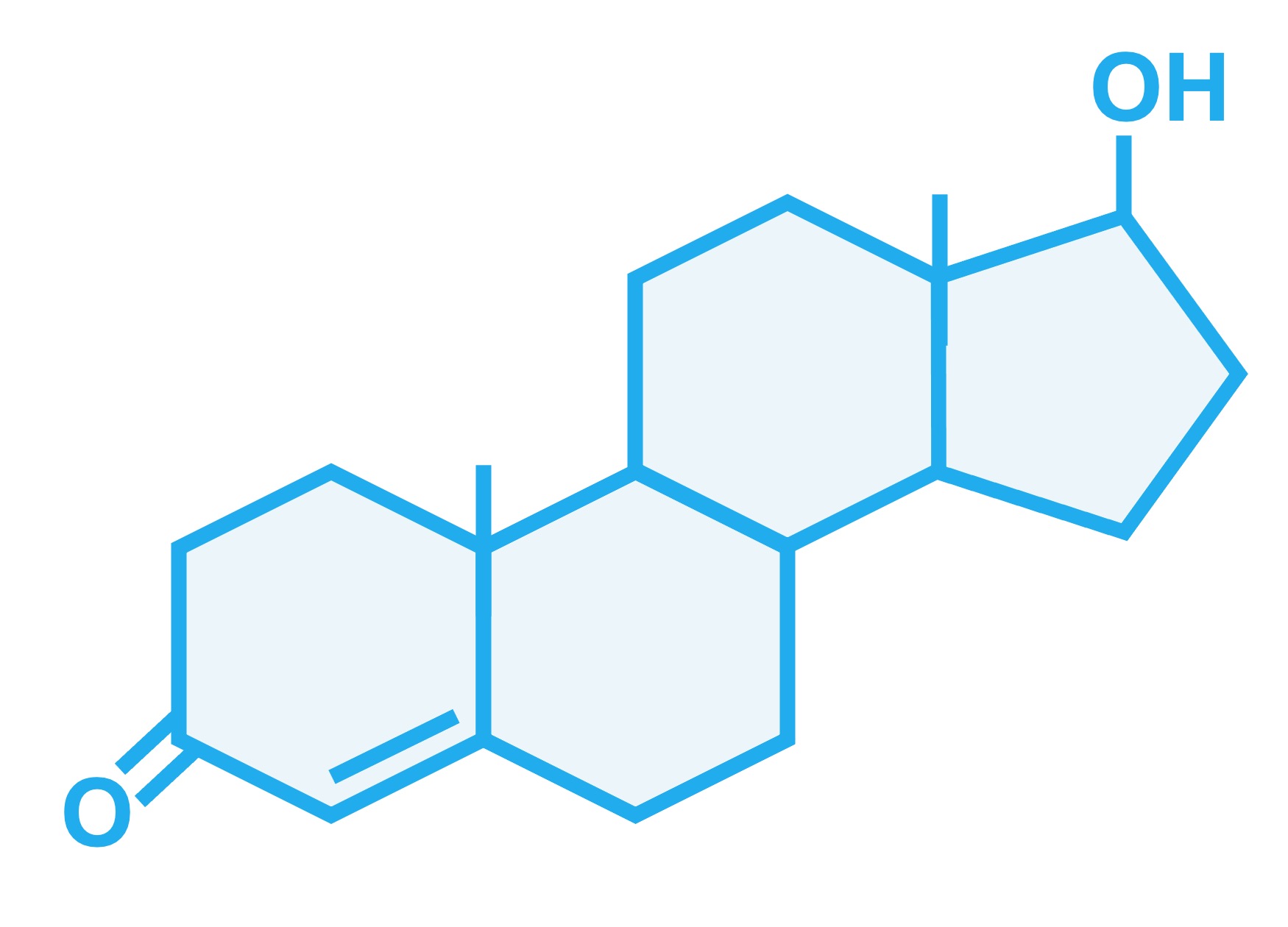
Additionally, lipophilic molecules (such as non-polar steroids like oestradiol and testosterone) can freely cross the bilayer
Large and charged molecules (such as ions and polar molecules) cannot cross the membrane via simple diffusion
Non-Polar Steroids
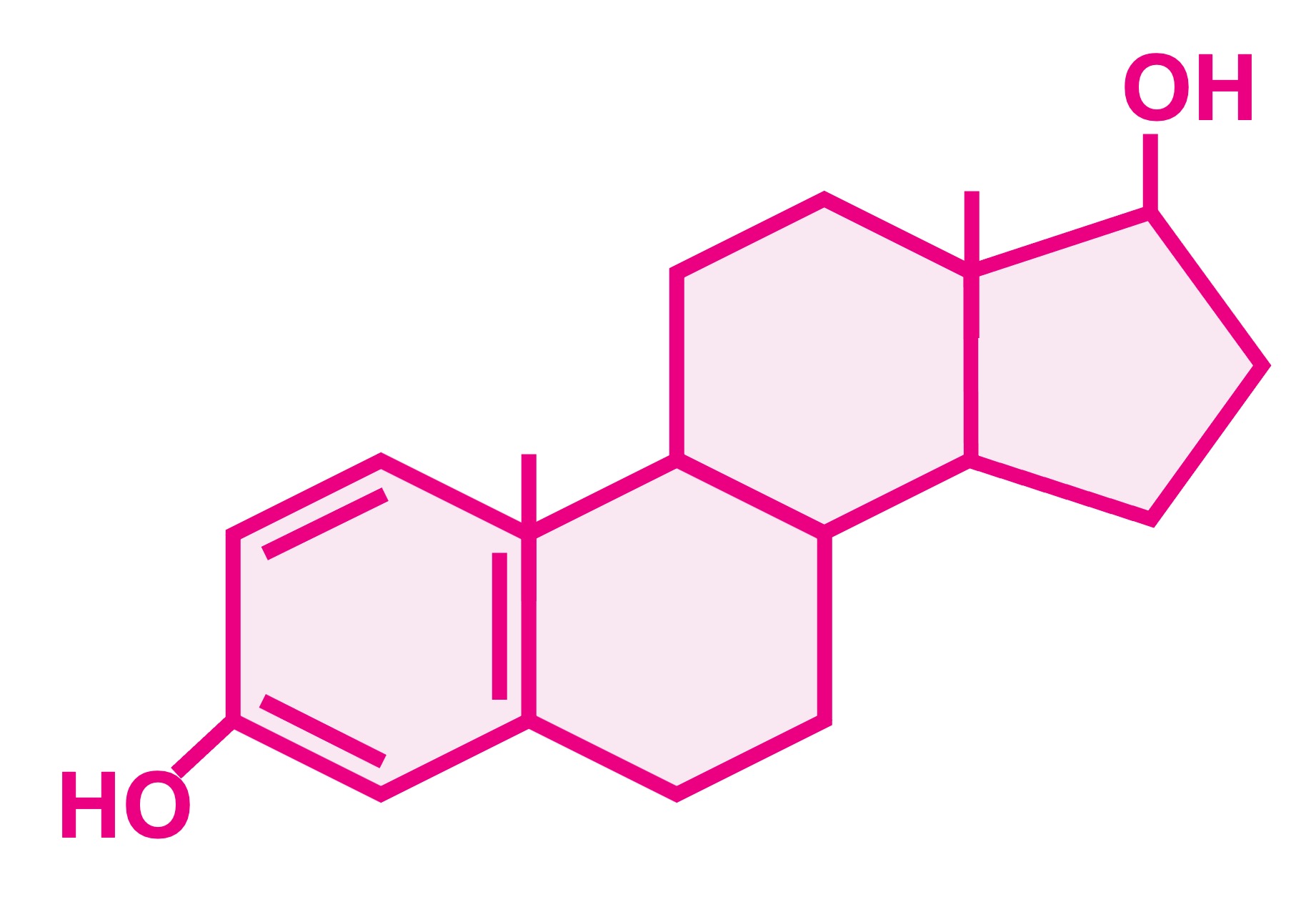
Oestradiol