Protein Modification
Certain proteins may need to be modified after being translated in order to assume a final functional state
-
These post-translational modifications involve a variety of covalent changes to the protein, including:
-
The formation of disulphide bridges between cysteine residues
-
Conjugation with other proteins or inorganic cofactors (e.g. the haeme group in haemoglobin)
-
Chemical modifications (such as glycosylation or phosphorylation) to improve structural stability or moderate biological activity
-
The removal of amino acids from the polypeptide chain (proteolytic cleavage) to mediate protein folding
-
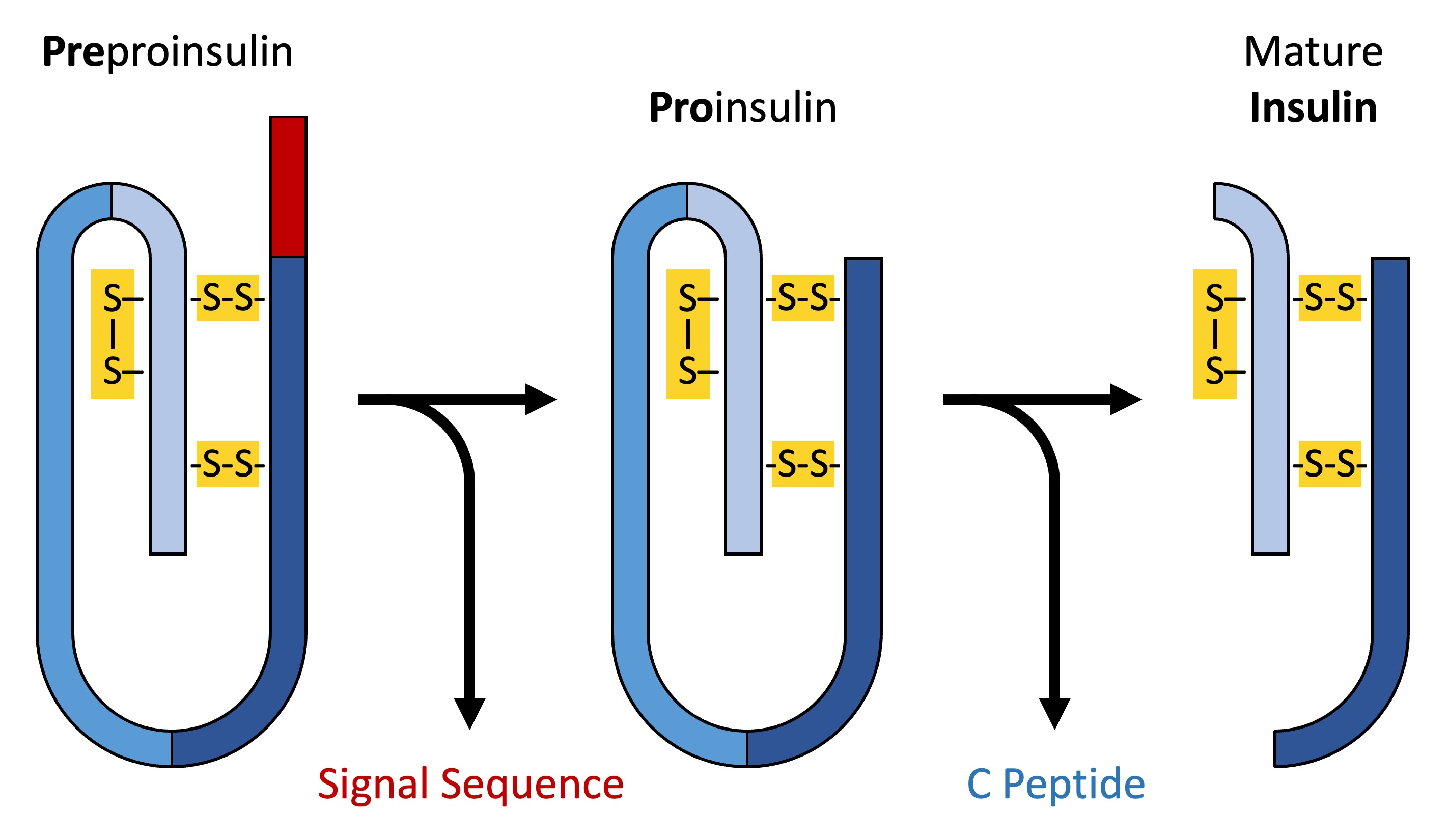
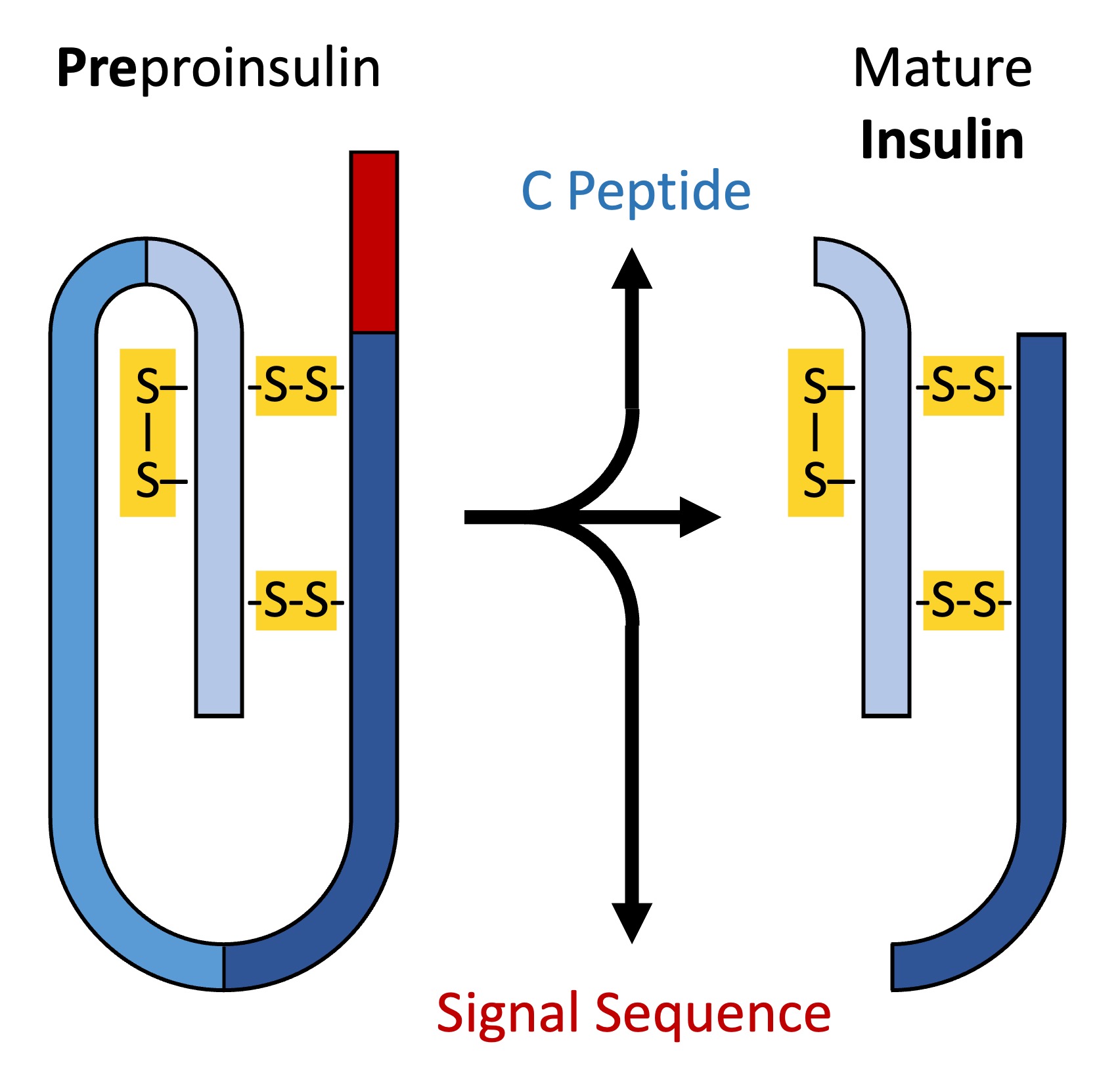
An example of a protein that requires modification before it can function is human insulin
-
Insulin is synthesised as an inactive precursor molecule called pre-proinsulin and undergoes a two-step modification process
-
Preproinsulin is converted into proinsulin when a signal sequence is removed in the rough endoplasmic reticulum (the signal sequence directed the ribosome to translate the protein directly into the rough ER)
-
As the proinsulin folds within the Golgi complex, opposite ends of the protein (the A and B chains) are linked by disulphide bridges – the intervening segment (called the C peptide) is removed
-
The resulting mature insulin is then packaged into secretory vesicles and stored until use (regulatory secretion)
-
Insulin Modification