Protein Destinations
All proteins produced by eukaryotic cells are initially synthesised by ribosomes found freely circulating within the cytosol
-
If the protein is targeted for intracellular use within the cytosol, the ribosome remains free and unattached
-
If the protein is targeted for secretion, membrane fixation or use in lysosomes, the ribosome becomes bound to the ER
Rough ER
Protein destination is determined by the presence or absence of an initial signal sequence on a nascent polypeptide chain
-
The presence of this signal sequence results in the recruitment of a signal recognition particle (SRP), which halts translation
-
The SRP-ribosome complex then docks at a receptor located on the ER membrane (forming rough ER)
-
Translation is re-initiated and the polypeptide chain continues to grow via a transport channel into the lumen of the ER
-
The signal sequence is cleaved and the SRP recycled once the polypeptide is completely synthesised within the ER
ER Targeting
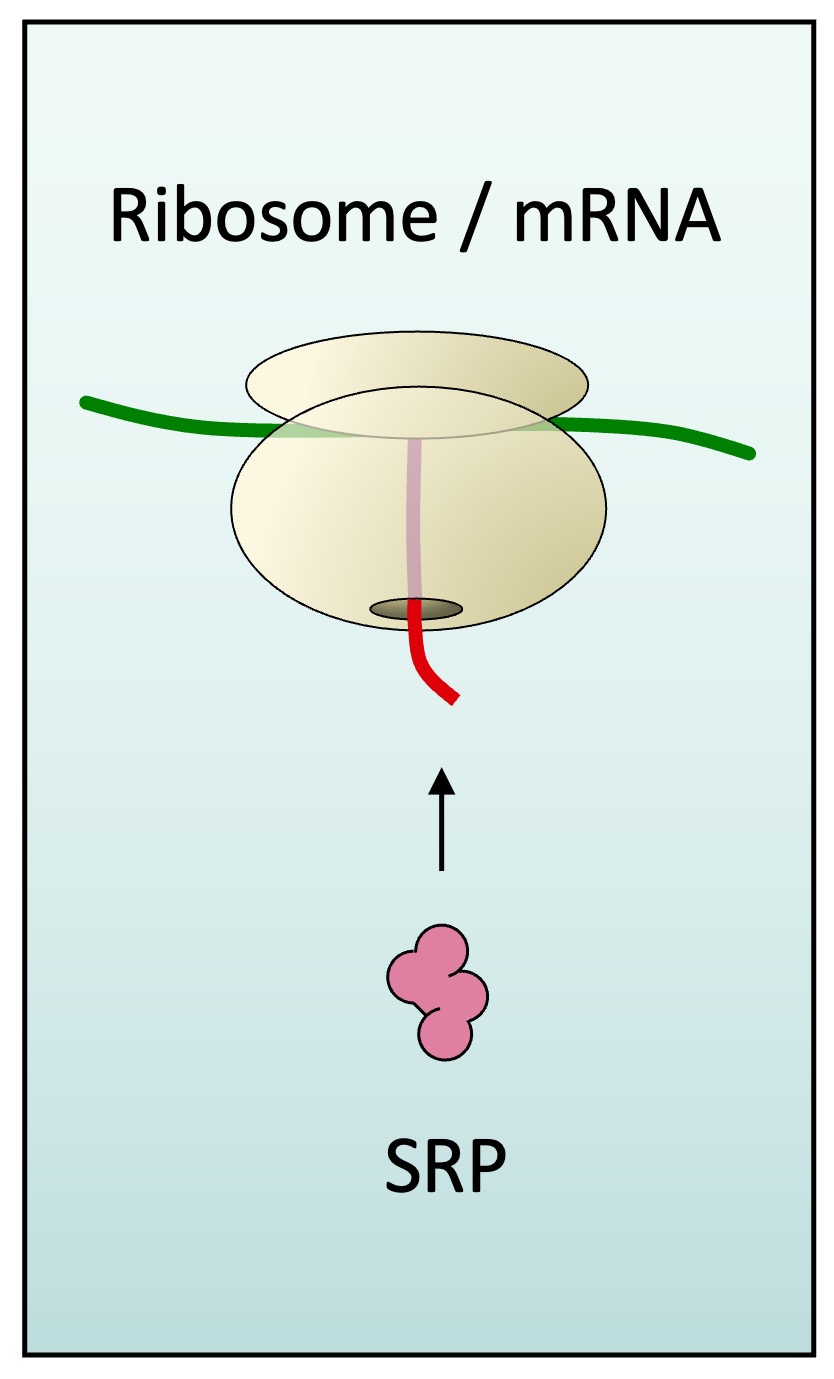
Detection
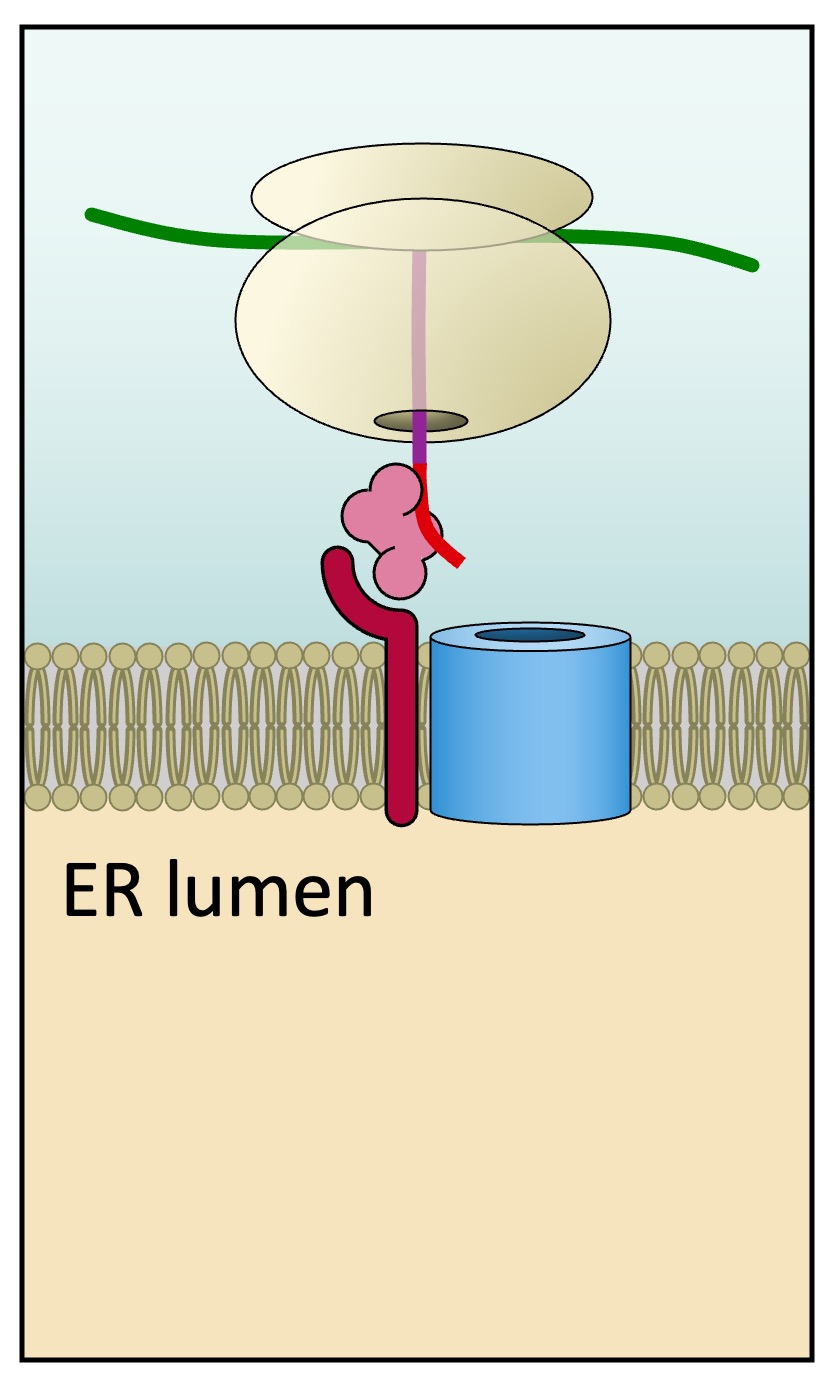
ER Transport
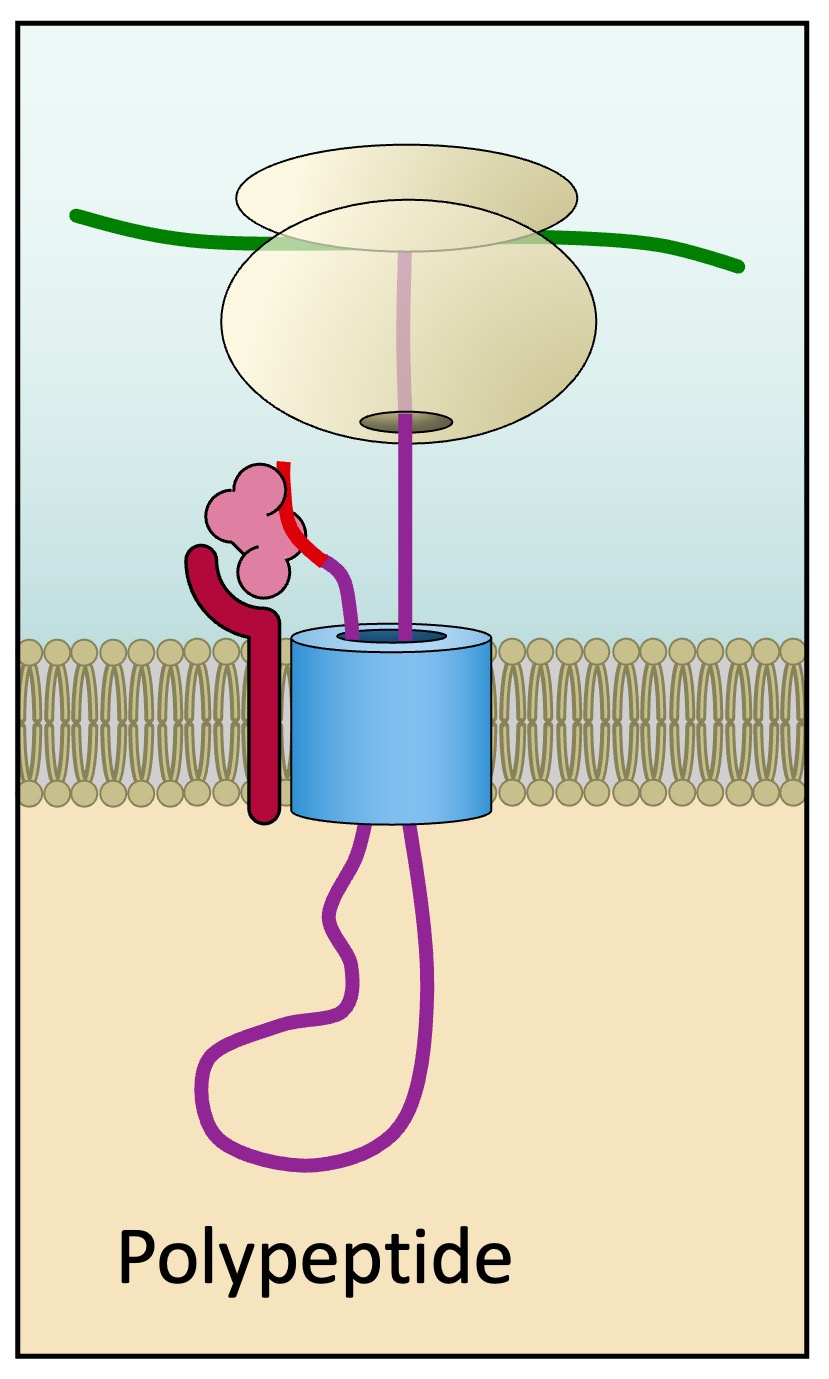
Internalisation
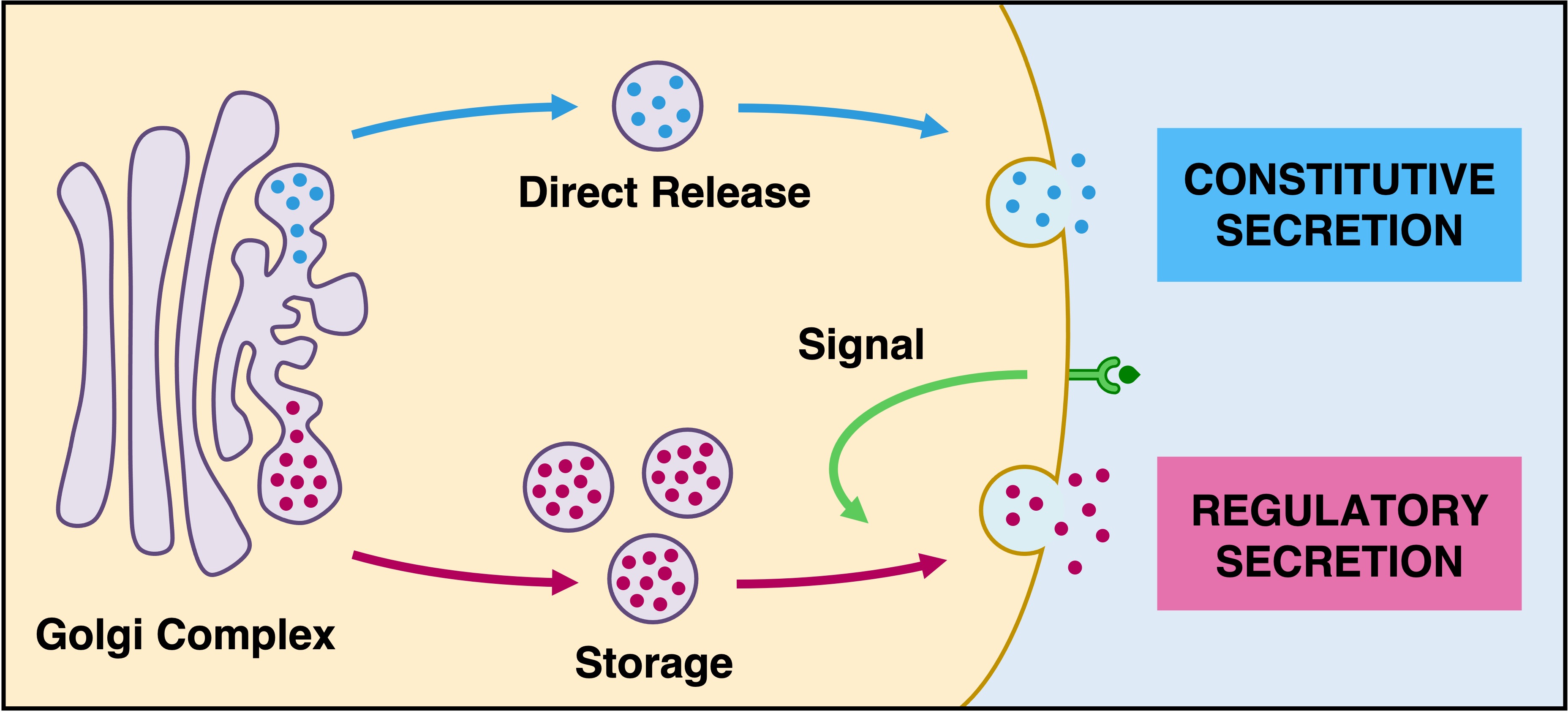
Golgi Complex
The Golgi apparatus is responsible for sorting, storing, modifying and exporting proteins from the cell
-
Proteins from rough ER arrive in vesicles at the Golgi body and are modified into functional molecules
-
The different sacs of the Golgi are responsible for specific chemical modifications (e.g. phosphorylation, glycosylation, etc.)
Proteins destined for secretion are packaged into vesicles at the Golgi body for extracellular release (exocytosis)
-
These materials can be either released immediately (constitutive secretion) or stored in secretory vesicles for a sustained release (regulatory secretion)
-
Regulatory secretion is triggered by an external chemical signal (ligand) binding to a specific receptor
Protein Secretion