Osmosis
Osmosis is a special form of simple diffusion that involves the movement of free water molecules
-
While water is a polar molecule, it is small enough to move between phospholipids in the bilayer
-
As a polar molecule, it is attracted to, and will associate with, other polar molecules or charged ions
-
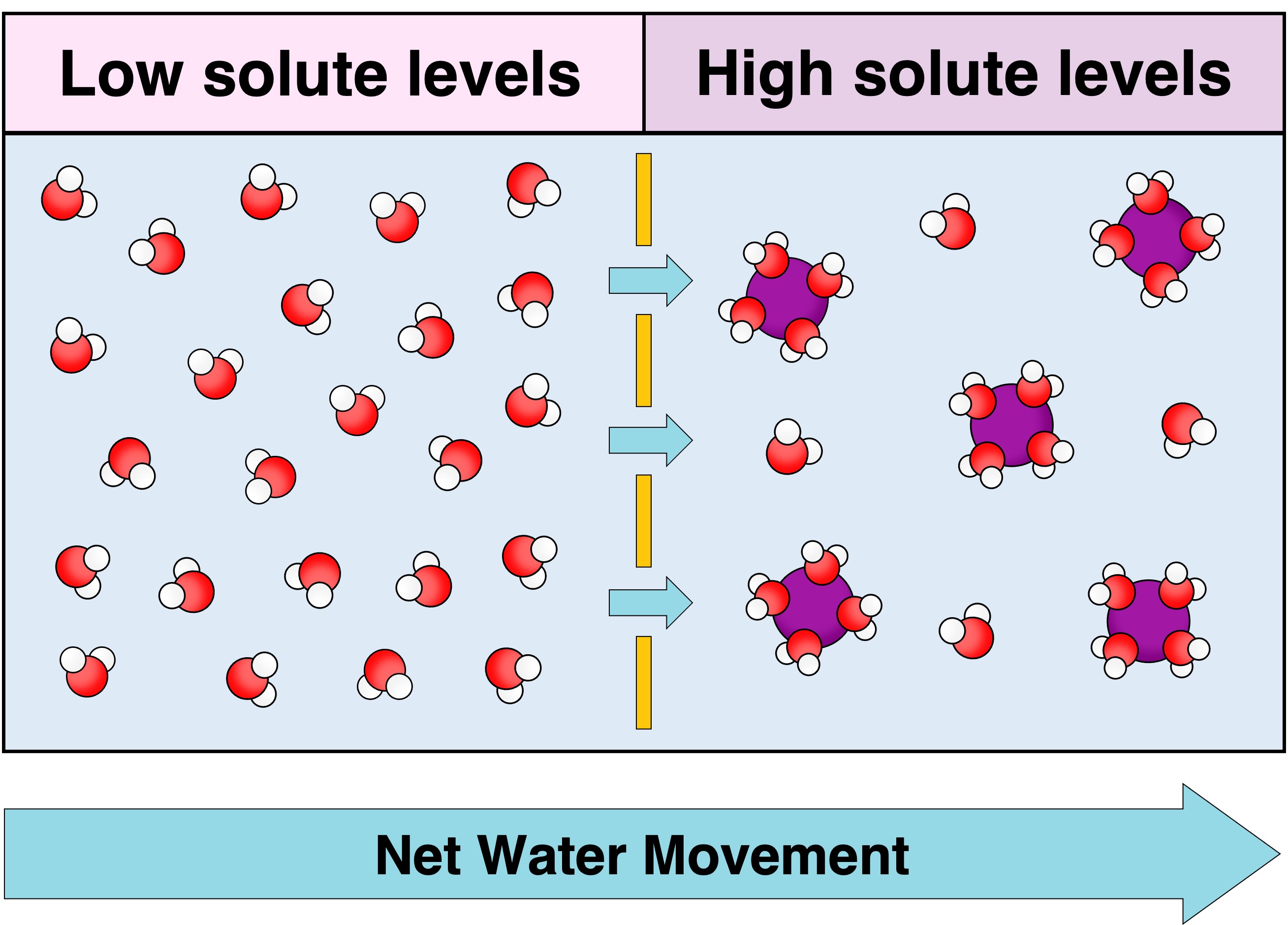
Water will therefore move towards the solution containing a higher concentration of ions or polar molecules
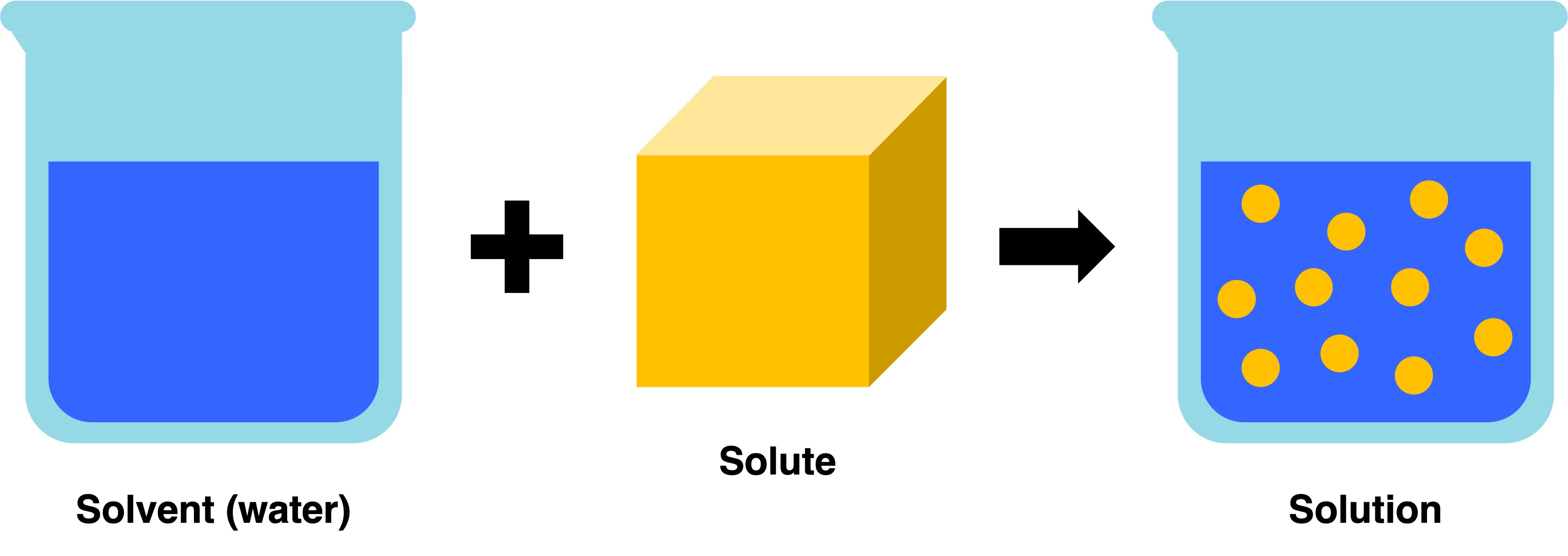
Water is a solvent capable of dissolving any polar or charged molecule (solute) to create a solution
Solute vs Solvent
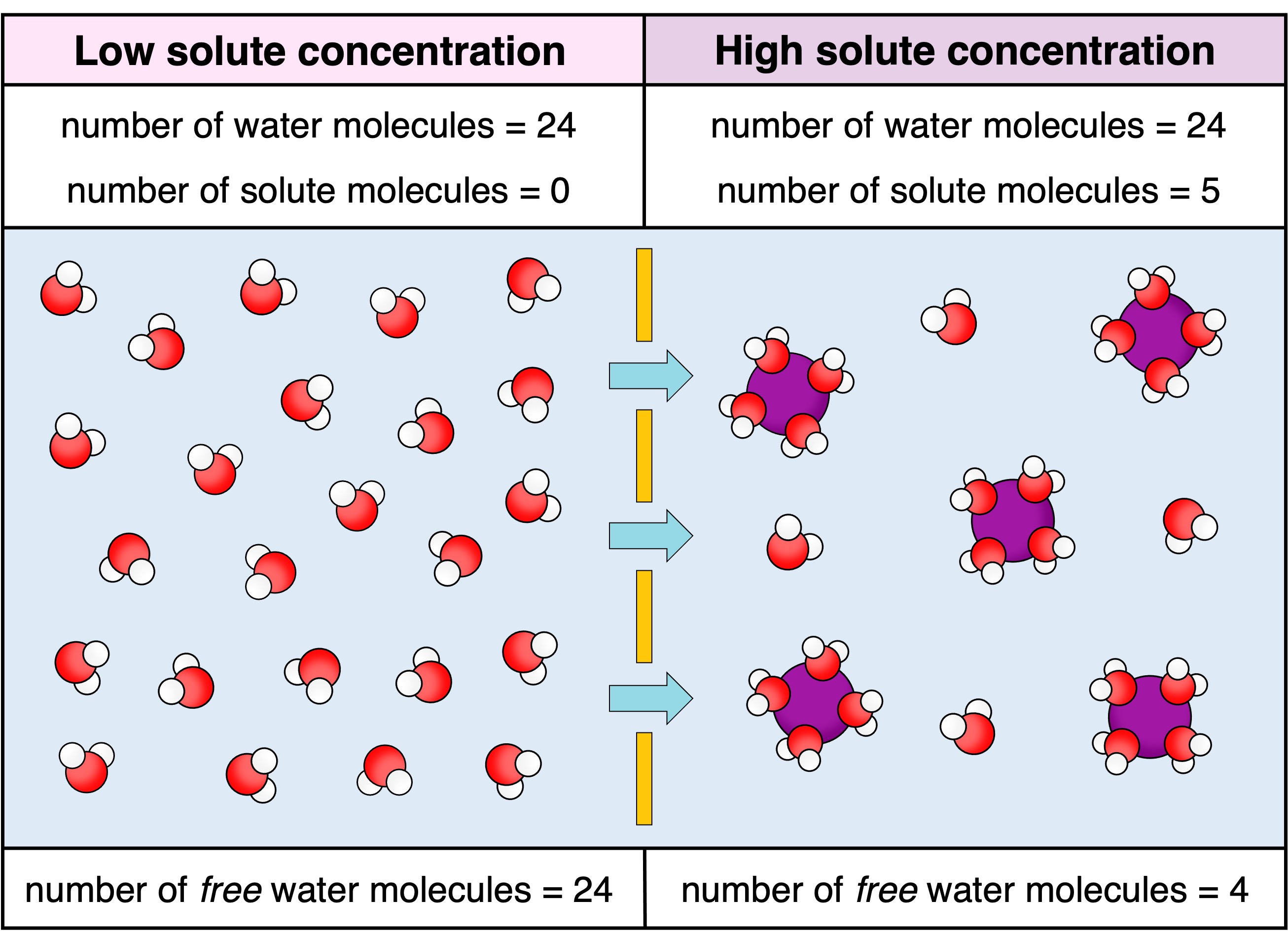
Osmosis is the net movement of water molecules across a semi-permeable membrane from a region of low solute concentration to a region of high solute concentration (until equilibrium is reached)
-
While water is moving to a more concentrated solution, osmosis is still a passive process as there are less free water molecules when solute levels are higher (so water is still moving along the water gradient)
Cell membranes also possess integral proteins called aquaporins that function as water channels within the bilayer
-
Aquaporins facilitate a much faster rate of water transport in response to solute concentrations and their levels can be regulated to help control the osmotic conditions of a cell
Osmosis