Monosaccharides
Carbohydrates are made of C, H and O (‘carbo’ – contains carbon ; ‘hydrate’ – contains H and O)
-
Carbohydrates are composed of recurring monomers called monosaccharides (‘mono’ – single ; 'saccharide’ – sugar)
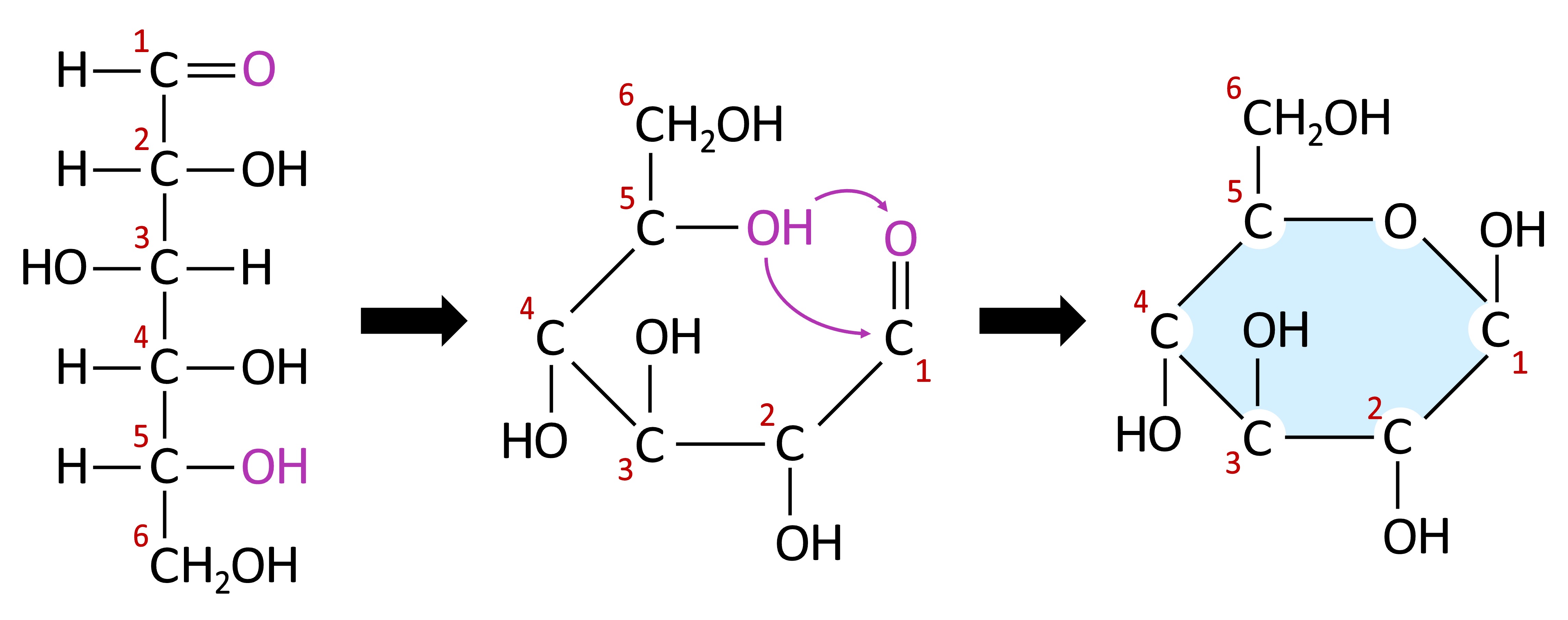
Monosaccharides typically form ring structures as a result of a chemical reaction between functional groups at opposite ends of the molecule
-
A hydroxyl group (-OH) links to a carbonyl group (=O) to form a cyclic structure connected by an oxygen atom
Monosaccharide Cyclization

Types of Monosaccharides
Most monosaccharides have either 5 carbons (pentose sugars) or 6 carbons (hexose sugars)
-
The name describes the number of carbons – not the shape (e.g. fructose is a hexose sugar but forms a pentagon)
An example of a pentose sugar is ribose – which is a core component of RNA nucleotides and is also found in coenzymes (such as ATP)
-
DNA nucleotides have a modified form of this pentose sugar in which an oxygen atom is removed (deoxyribose)
An example of a hexose sugar is glucose – which is primarily used as a source of energy (it is digested via cell respiration to produce ATP)
-
Glucose can exist as one of two isomers (⍺-D glucose or ß-D glucose) depending on the orientation of the 1’-OH group
Pentose vs Hexose Sugars
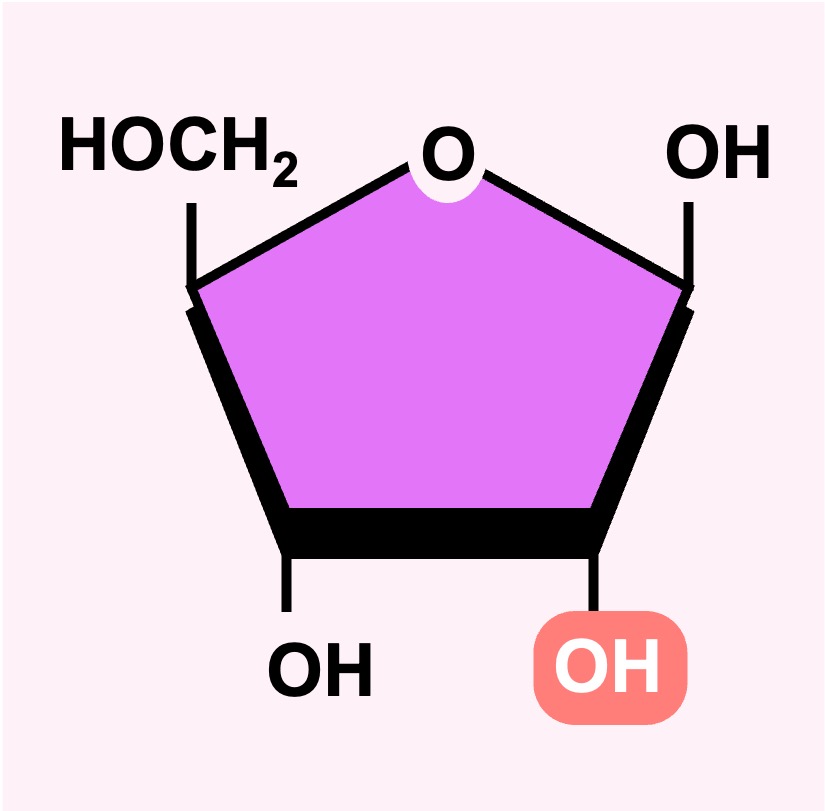
Ribose

Deoxyribose
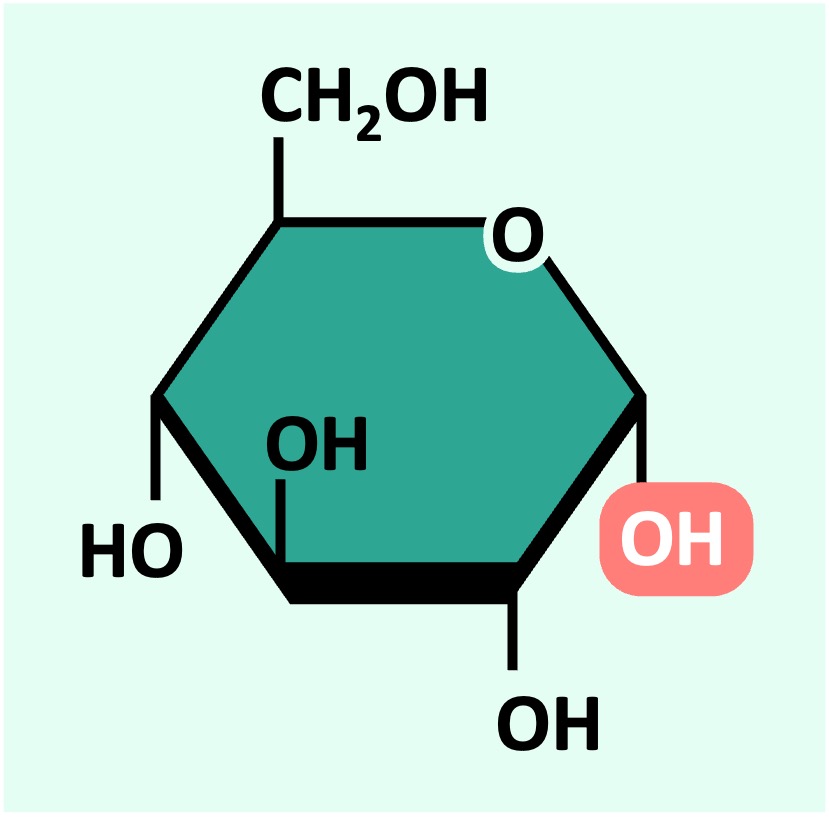
⍺-D glucose
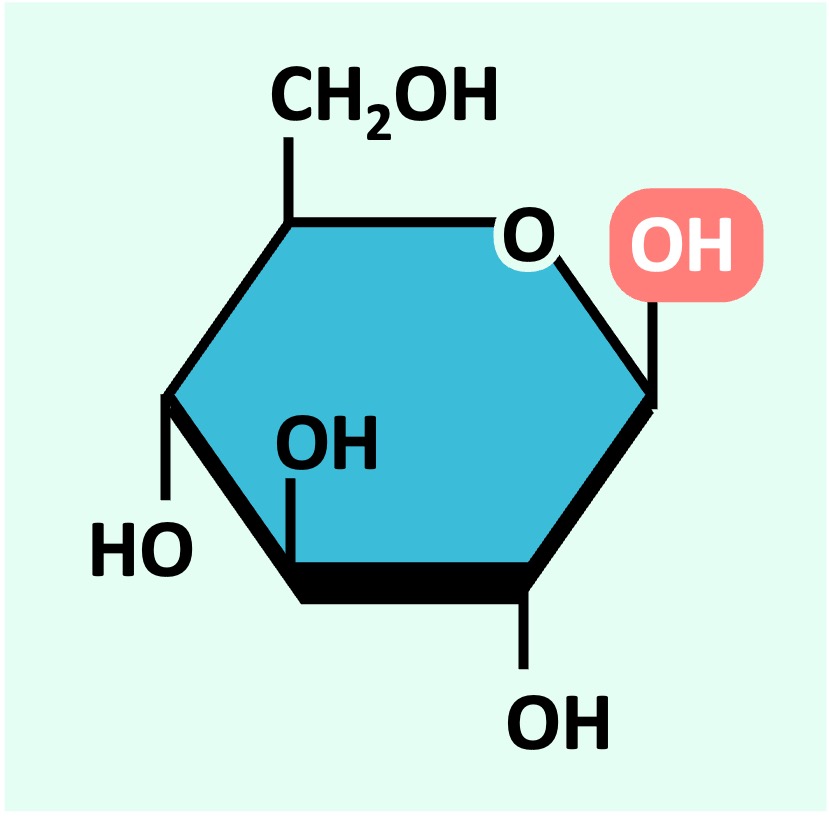
ß-D glucose
Function of Monosaccharides
The primary role of most monosaccharides is to function as a source of energy for the cell
-
Monosaccharides are oxidised (broken down) to produce large quantities of biological energy (ATP) via cellular respiration
Glucose is the most common monosaccharide to be used as an energy source due to its various chemical properties:
-
Solubility: Glucose is a polar molecule (due to –OH groups) and so will dissolve in water (it is hydrophilic)
-
Stability: Glucose is a very stable molecule as cyclic structures are generally more energetically favourable than straight chains
-
Transport: Because glucose is soluble and stable, it is easier to transport within aqueous solutions (like blood or cytosol)
-
Potential Energy: Glucose has many high energy electrons (between C–C and C–H bonds) which can be released via oxidation
-
ATP Yield: Glucose can by oxidised to produce a large yield of ATP via aerobic cell respiration