Hydrogen Carriers
When organic compounds such as glucose are broken down by cellular respiration, the chemical energy that is released can be transferred to one of two coenzyme molecules
-
Coenzymes are molecules that facilitate energy transfer by cycling between a loaded and unloaded state
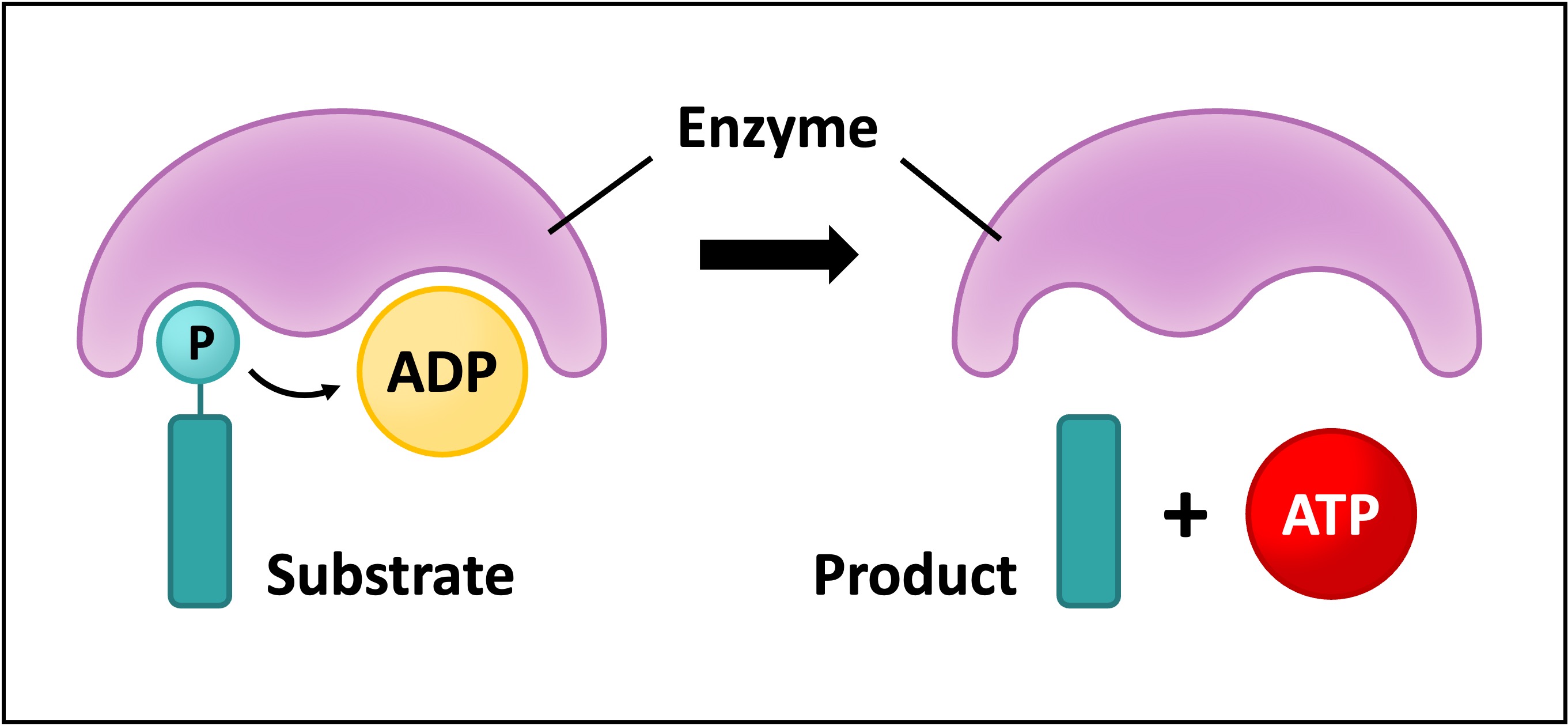
ATP is the primary energy carrier of the cell and can be directly produced via substrate-level phosphorylation
-
This involves an enzyme using the energy released from a phosphorylated organic compound (substrate) to fuse the phosphate to an unloaded ADP
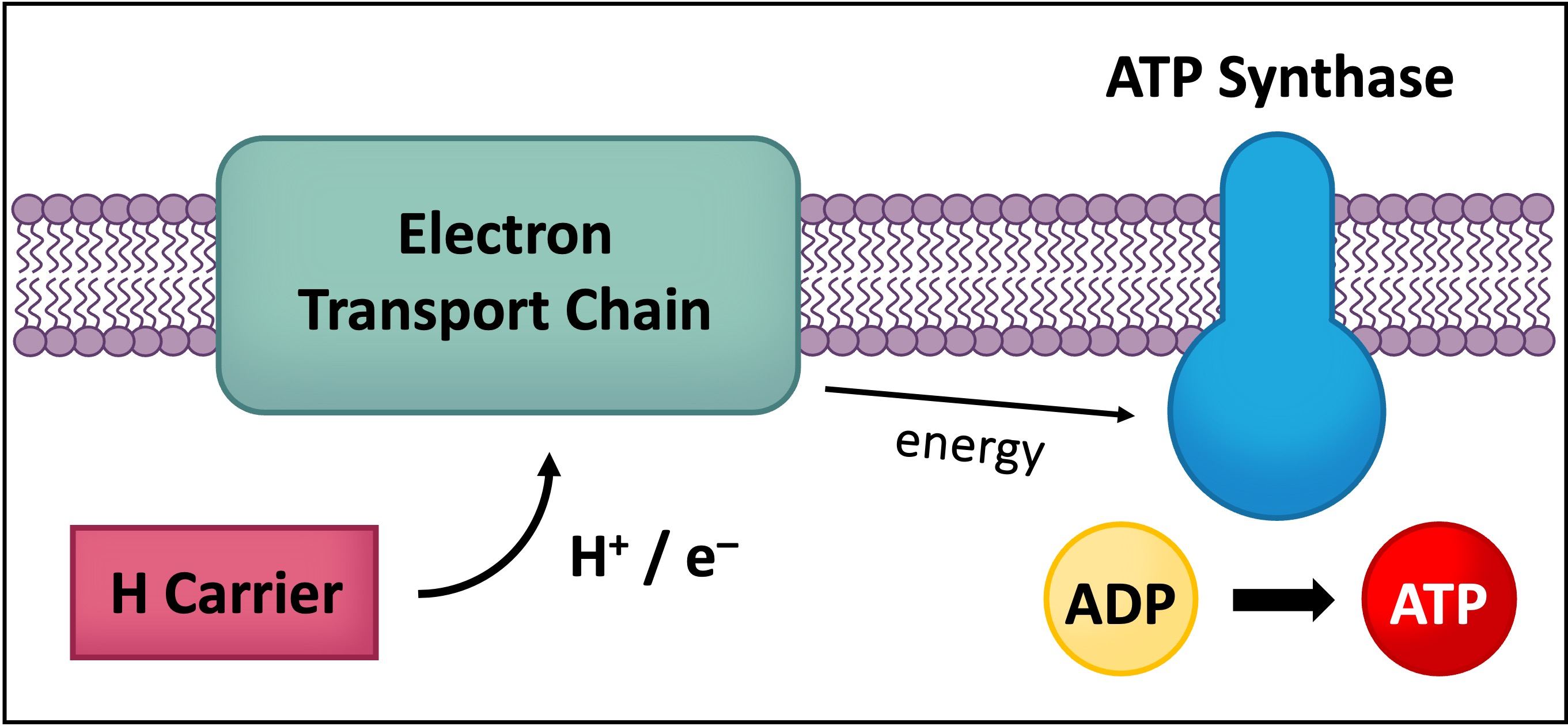
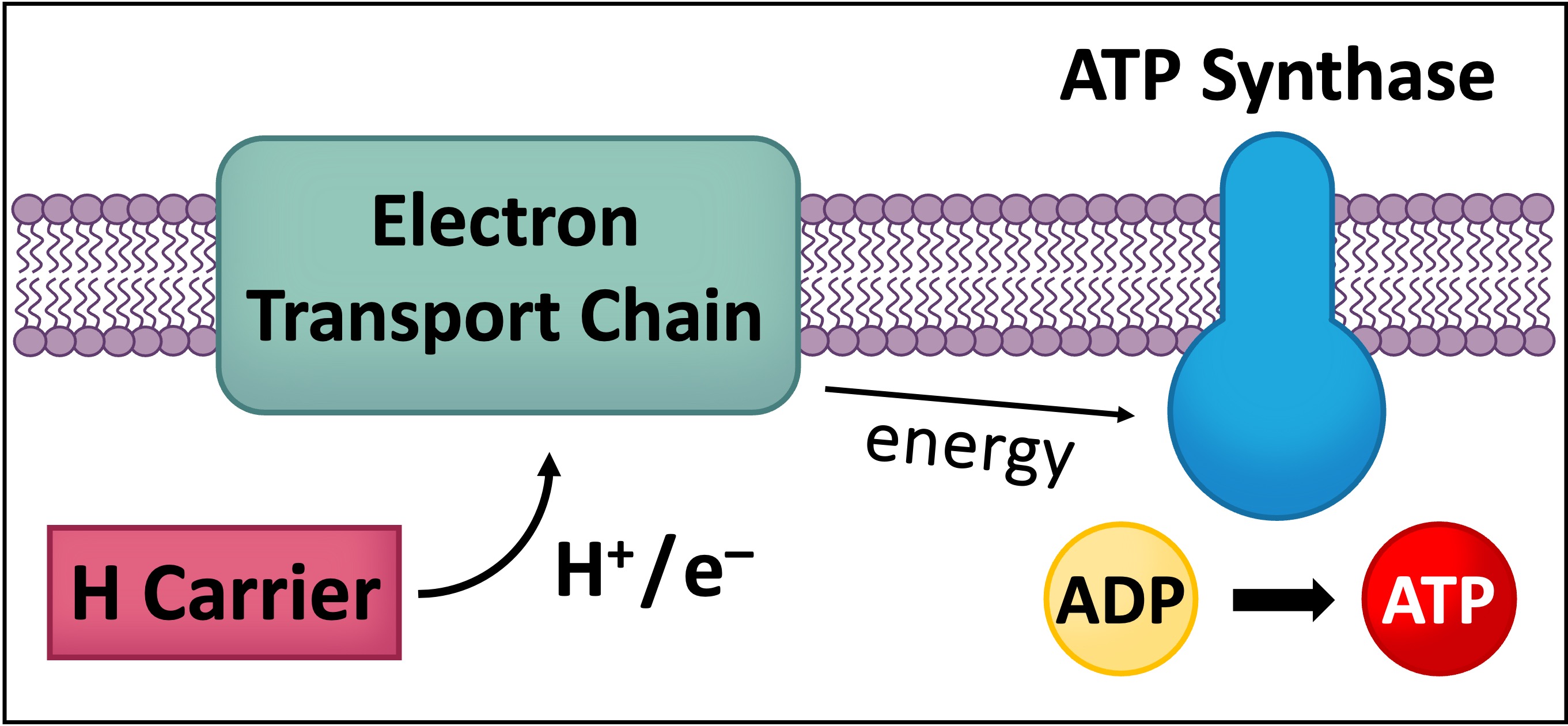
Hydrogen carriers act as a transitional energy carrier and can indirectly transfer energy to form ATP via oxidative phosphorylation
-
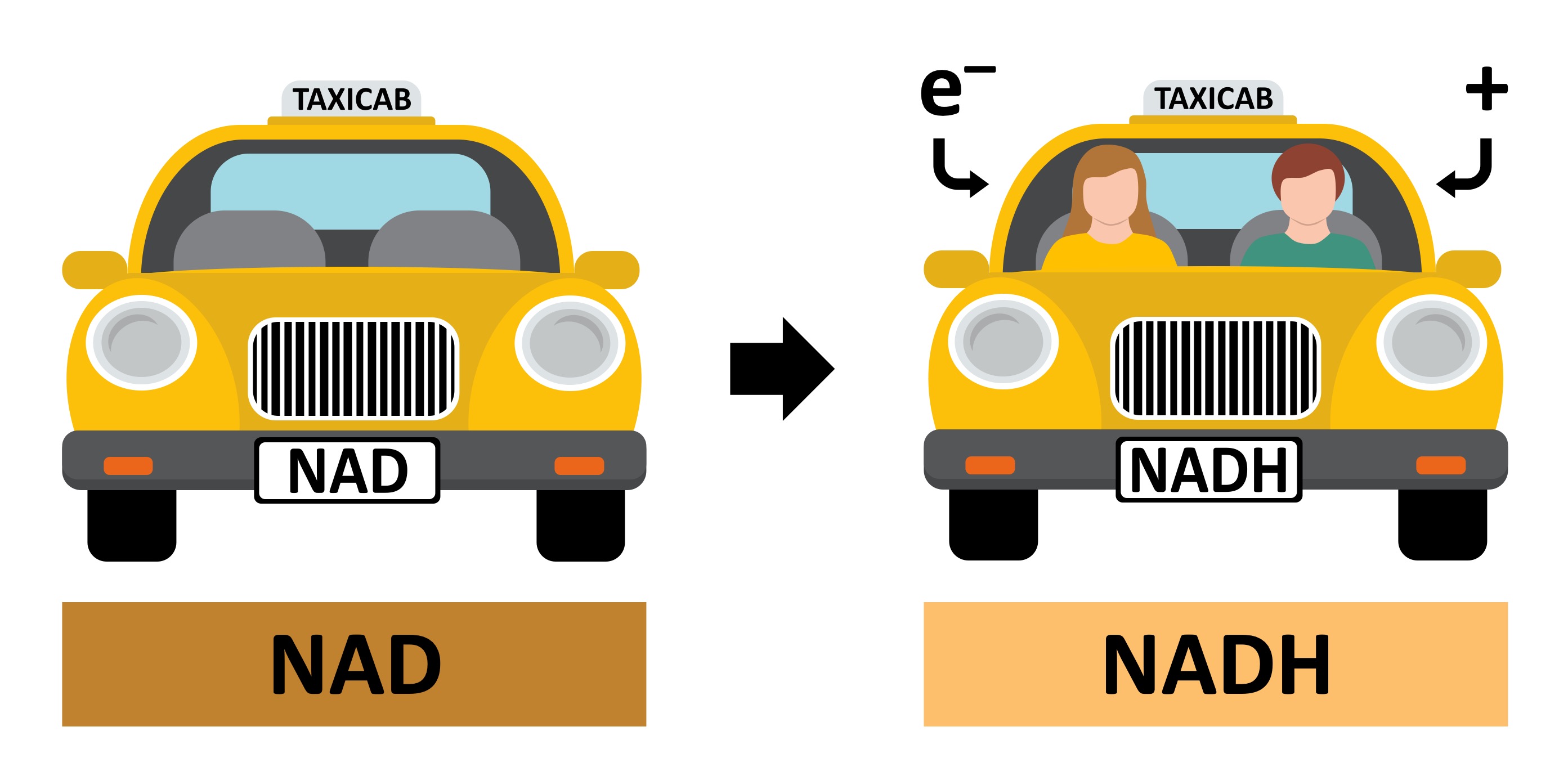
Hydrogen atoms (released from the organic compound) consist of protons and high energy electrons
-
These high energy electrons can be transferred by the hydrogen carrier to an electron transport chain in the mitochondria (via oxidation)
-
The energy from the electrons can be used to synthesise ATP in a process that requires oxygen (hence oxidative phosphorylation can only occur via aerobic respiration)
Methods of ATP Production

Substrate Level
Oxidative
Redox Reactions
When organic molecules are broken down by cell respiration, the compounds released are transferred by means of redox reactions
-
Redox reactions involved the reduction of one chemical species and the oxidation of another (redox = reduction / oxidation)
Most redox reactions typically involve the transfer of electrons, hydrogen or oxygen
-
Reduction is the gain of electrons / hydrogen or the loss of oxygen (the oxidising agent is reduced)
-
Oxidation is the loss of electrons / hydrogen or the gain of oxygen (the reducing agent is oxidised)
Redox reactions can be summarised as follows:
-
OIL RIG – Oxidation Is Loss (of electrons) ; Reduction Is Gain (of electrons)
-
LEO goes GER – Loss of Electrons is Oxidation ; Gain of Electrons is Reduction
-
ELMO – Electron Loss Means Oxidation
-
Oxidation
Oxidise
Reduction
Reduce
Electron
Loss
Gain
Hydrogen
Loss
Gain
Oxygen
Gain
Loss
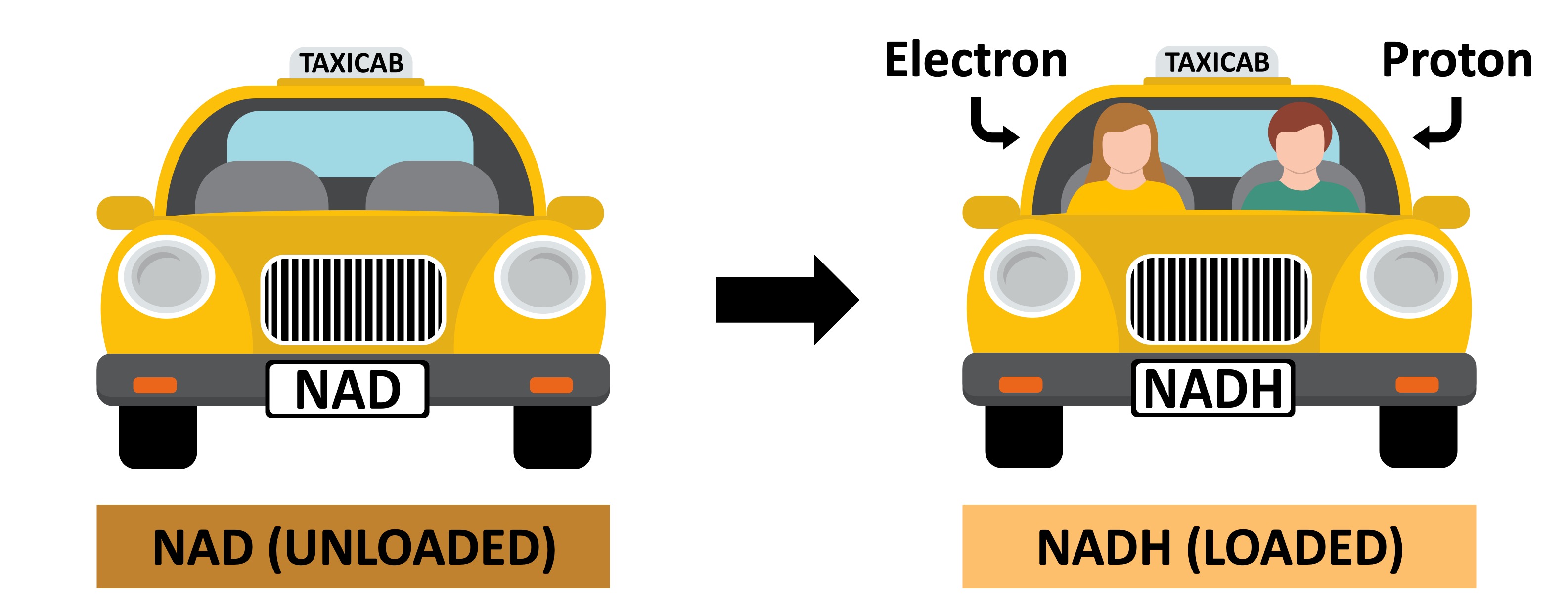
Hydrogen Carriers
The coenzymes that transport hydrogen atoms (electrons and protons) are collectively called hydrogen carriers
-
The most common hydrogen carrier is NAD which is reduced to form NADH
-
A less common hydrogen carrier is FAD which is reduced to form FADH2
The hydrogen carriers function like taxis, transporting the hydrogen atoms to the cristae of the mitochondria
-
At the cristae, the electrons and protons of the hydrogen atom are donated to the electron transport chain
-
Through this oxidation reaction, ATP is indirectly synthesised (via oxidative phosphorylation)
-
This process requires oxygen, so hydrogen carriers can only produce chemical energy via aerobic respiration
NAD → NADH