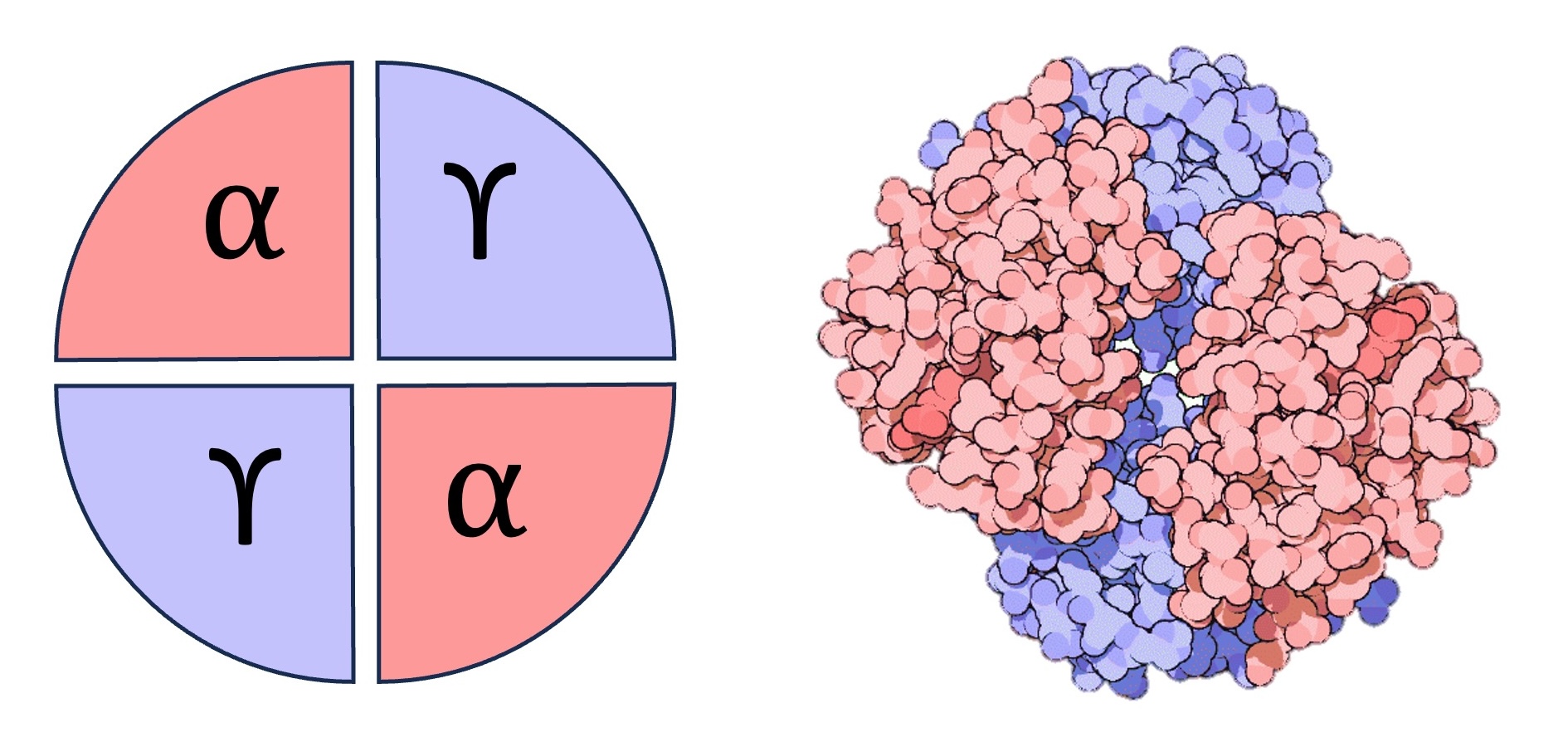Haemoglobin
Oxygen is transported throughout the body in red blood cells (erythrocytes), which contain an oxygen-binding protein called haemoglobin
-
Haemoglobin is a conjugated protein with a quaternary structure – it is composed of four polypeptide chains, each with an iron-containing heme group
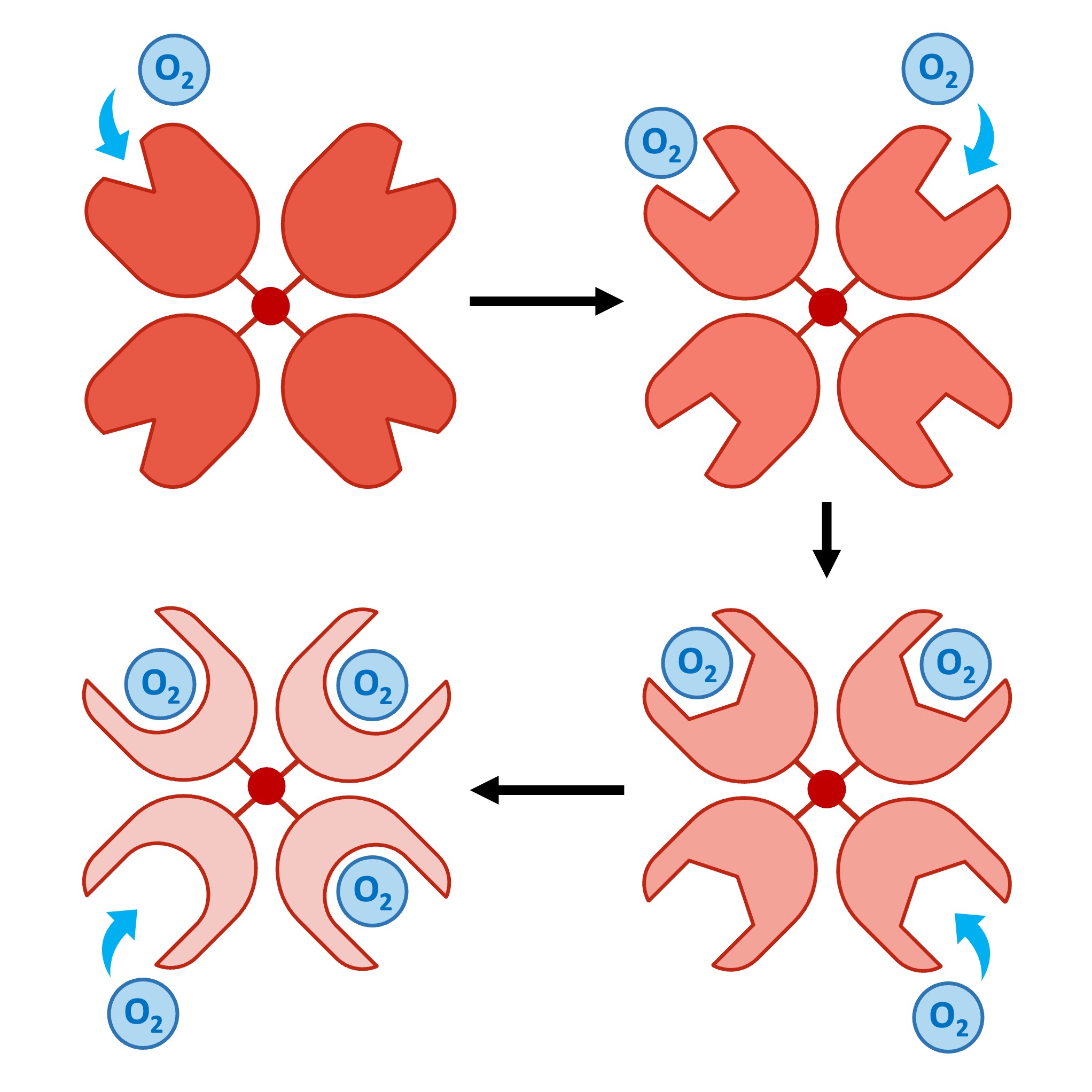
Each heme group can reversibly bind one oxygen molecule, meaning each haemoglobin can transport up to eight oxygen atoms (Hb + 4O2 = HbO8)
Oxygen Binding
As each oxygen molecule binds, it alters the conformation of haemoglobin, making subsequent binding easier (cooperative binding)
-
This means haemoglobin will have a higher affinity for O2 in oxygen-rich areas (like the lung), promoting oxygen loading
Additionally, carbon dioxide can also bind to haemoglobin via an allosteric site and cause a conformational change that lowers the affinity for oxygen
-
This means oxygen will be unloaded in oxygen-starved tissues (like muscles), where CO2 levels are elevated due to higher rates of cell respiration
Adult vs Foetal Haemoglobin
Humans produce different forms of haemoglobin before and after birth (foetal vs adult haemoglobin)
-
Adult haemoglobin is composed of two alpha chains and two beta chains, while foetal haemoglobin has gamma chains instead of beta chains
-
The gamma chains have a higher affinity for oxygen, meaning foetal haemoglobin will load oxygen when adult haemoglobin is unloading it
The different oxygen affinities ensures the foetus can obtain oxygen from the mother’s blood at the placenta (which acts as the mother-foetus interface)
-
Following birth, fetal haemoglobin is almost completely replaced by adult haemoglobin (~ 6 months post-natally)
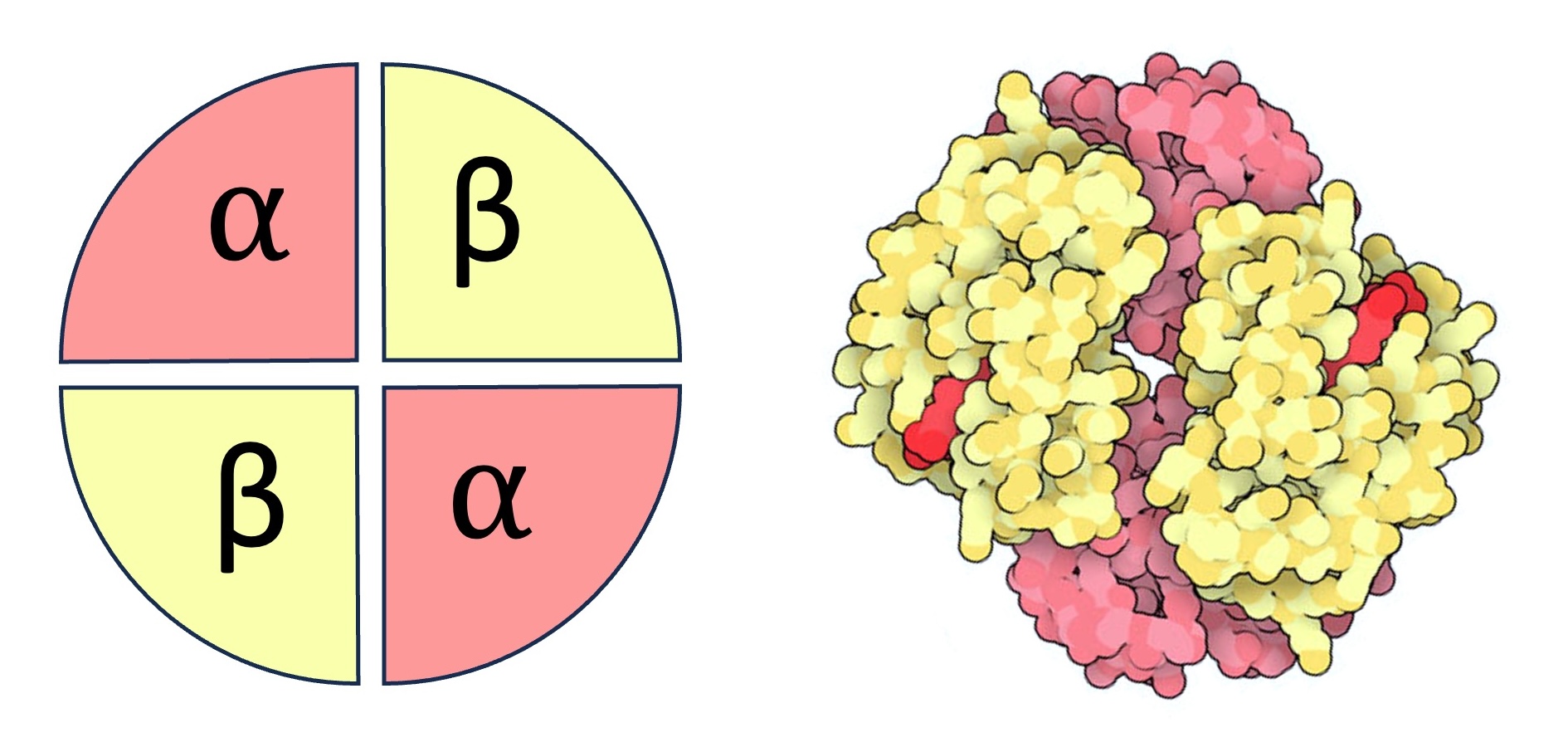
Adult Haemoglobin