Fermentation
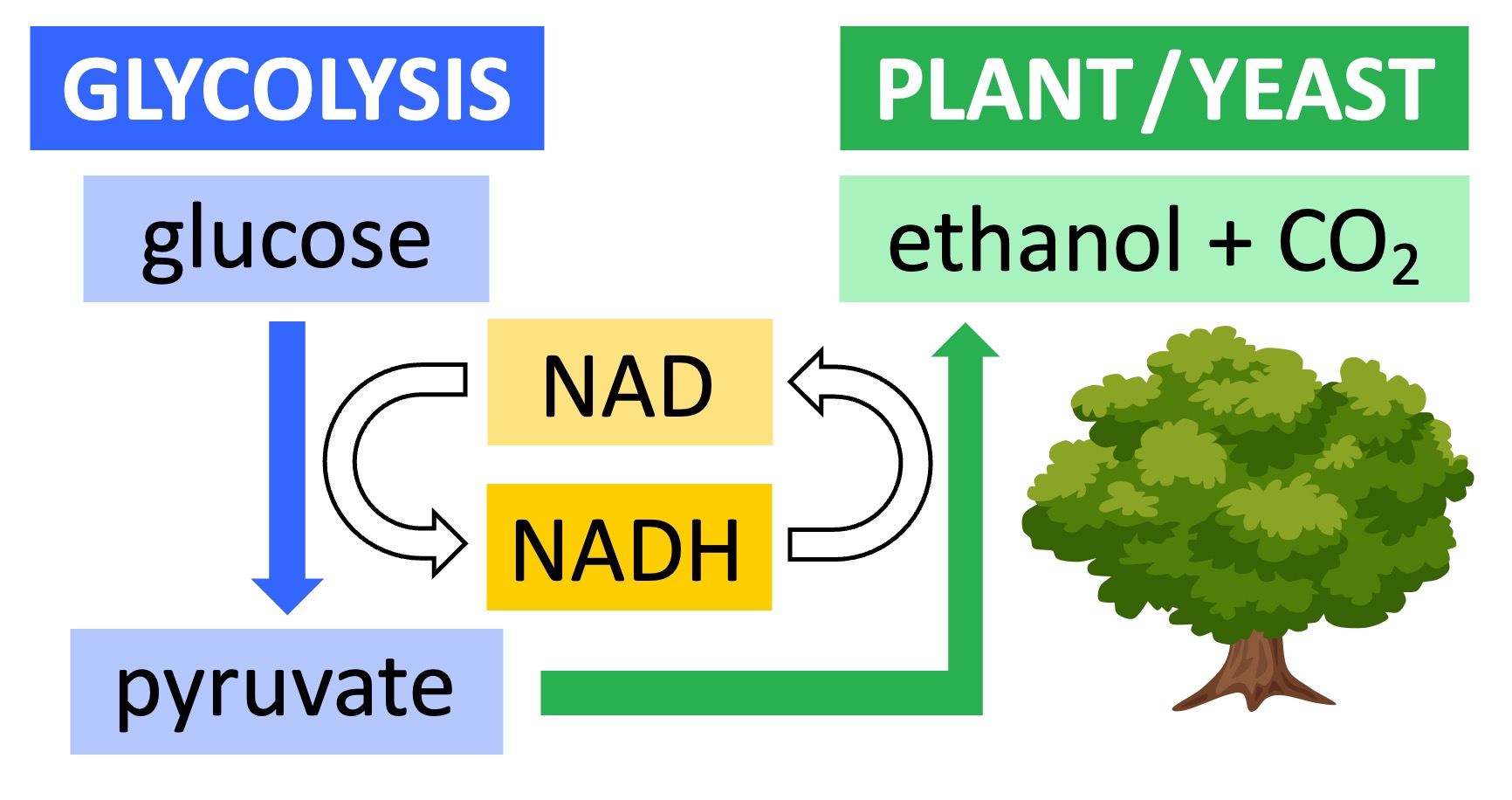
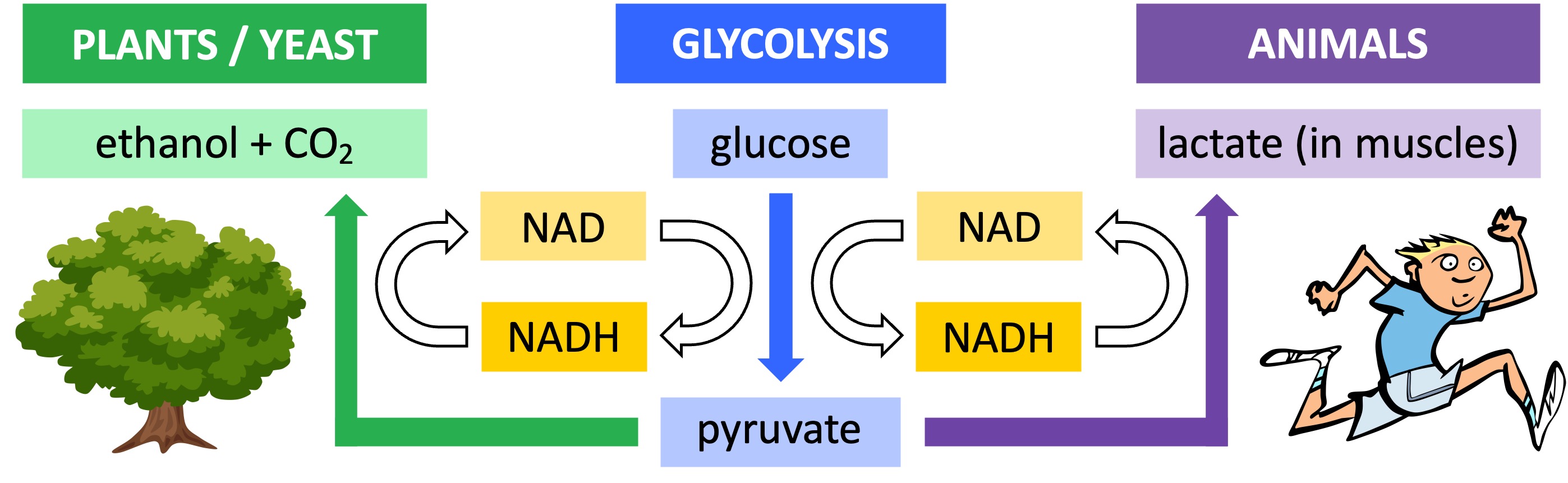
The anaerobic breakdown of glucose into pyruvate (via glycolysis) results in the production of a small amount of hydrogen carrier (NADH)
-
In the presence of oxygen, this hydrogen carrier will be oxidised as part of the electron transport chain (i.e. becomes unloaded NAD)
-
In the absence of oxygen, the carrier cannot be oxidised by mitochondria and stocks of the unloaded coenzyme will become depleted
Fermentation involves the conversion of pyruvate into an alternative carbon compound via a reaction that oxidises the hydrogen carrier
-
This restores the stocks of unloaded coenzyme needed for glycolysis, allowing ATP production to continue in the absence of oxygen
-
Fermentation is therefore considered to be a vital component of anaerobic respiration, as glycolysis could not otherwise be sustained
The products of fermentation differ between animals and plants or yeast:
-
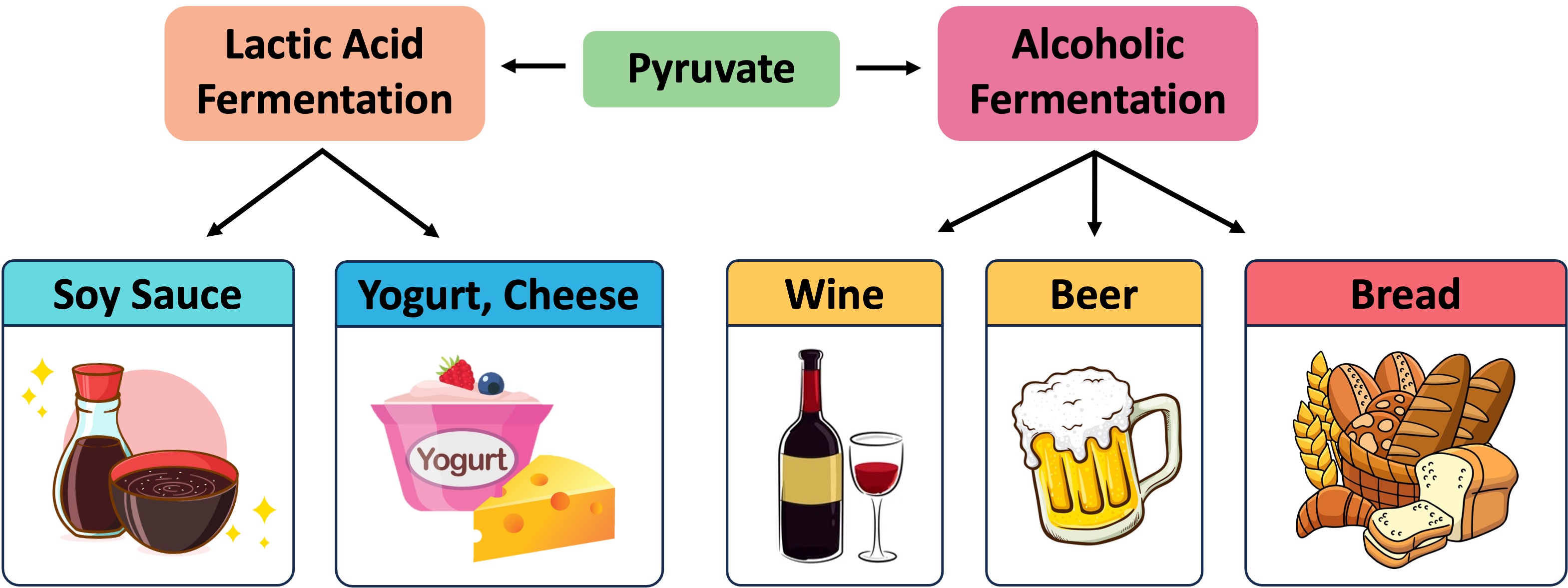
In animals, pyruvate is converted into lactic acid (lactate) – this reaction is reversible (pyruvate can be reformed when oxygen is present)
-
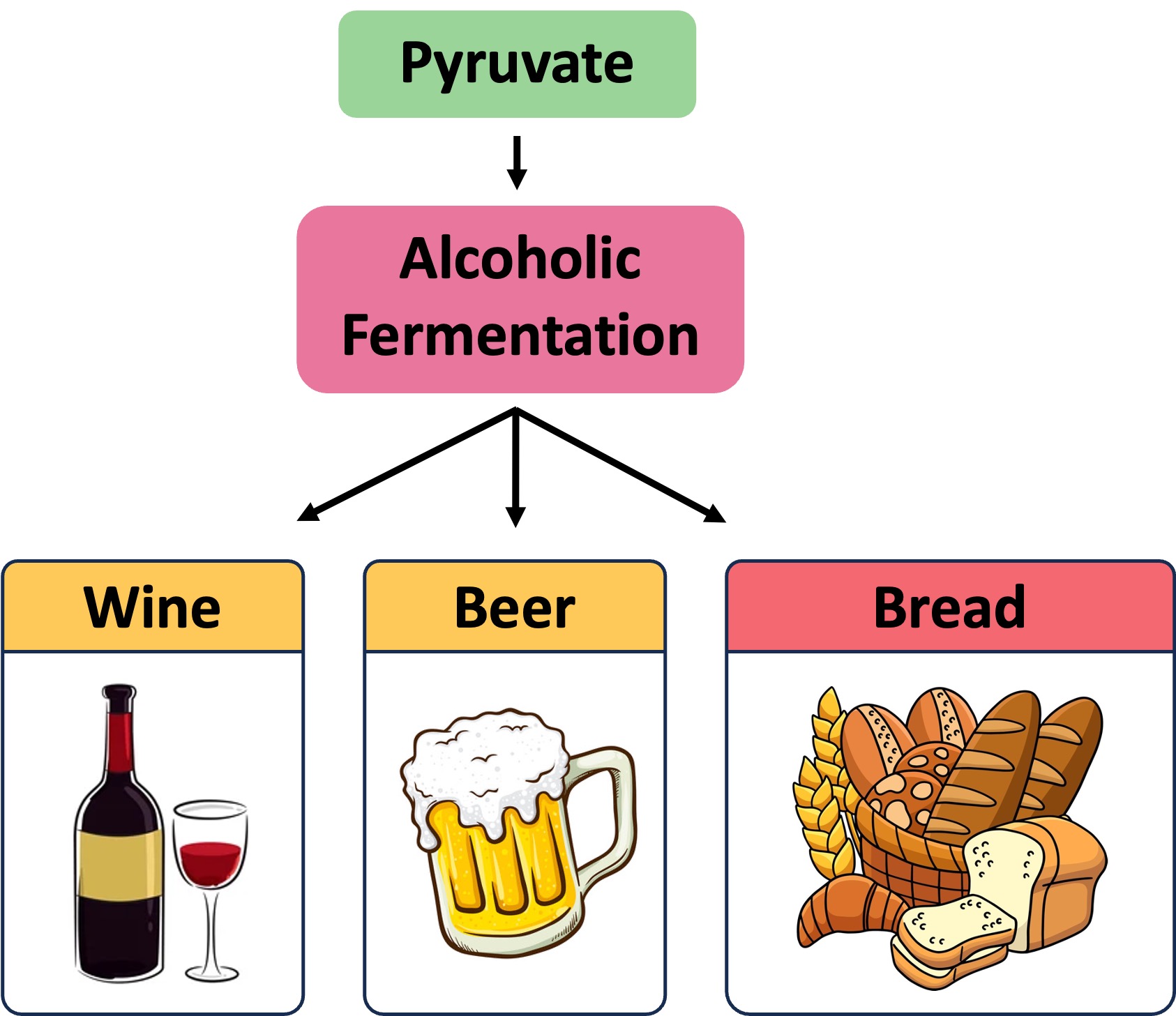
In plants or yeast, pyruvate is converted into ethanol and carbon dioxide – this reaction is irreversible (the pyruvate cannot be reformed)
Fermentation
Anaerobic fermentation has many practical applications:
-
In animals, fermentation is used to maximise the power of muscle contractions (allows energy capacity to exceed oxygen levels)
-
In bacteria, the lactic acid produced by fermentation can be used to modify milk proteins to make certain types of yogurts and cheese
-
The fermentation of organic matter can also result in the production of biofuels (e.g. bioethanol) – a renewable source of chemical energy
In yeasts, fermentation results in the production of ethanol and carbon dioxide – which can be used in food processing:
-
Bread – Carbon dioxide causes dough to rise via leavening (the ethanol evaporates during the baking process)
-
Alcohol – Ethanol is the intoxicating agent in alcoholic beverages (concentrations above ~14% damage the yeast)
Food Preparation