Enzyme Inhibition
Competitive Inhibition
Competitive inhibition involves a molecule, other than the substrate, binding to the enzyme’s active site
-
The molecule (inhibitor) is structurally and chemically similar to the substrate (hence able to bind to the active site)
-
The competitive inhibitor blocks the active site and thus prevents substrate binding
-
As the inhibitor is in competition with the substrate, its effects can be reduced by increasing substrate concentration
Statins are common cholesterol lowering drugs that function via competitive enzyme inhibition
-
Statins bind to the active site of the enzyme HMG-CoA reductase which forms part of the metabolic chain responsible for cholesterol synthesis
-
By blocking this metabolic chain, cholesterol production is reduced, minimising the health consequences associated with high cholesterol
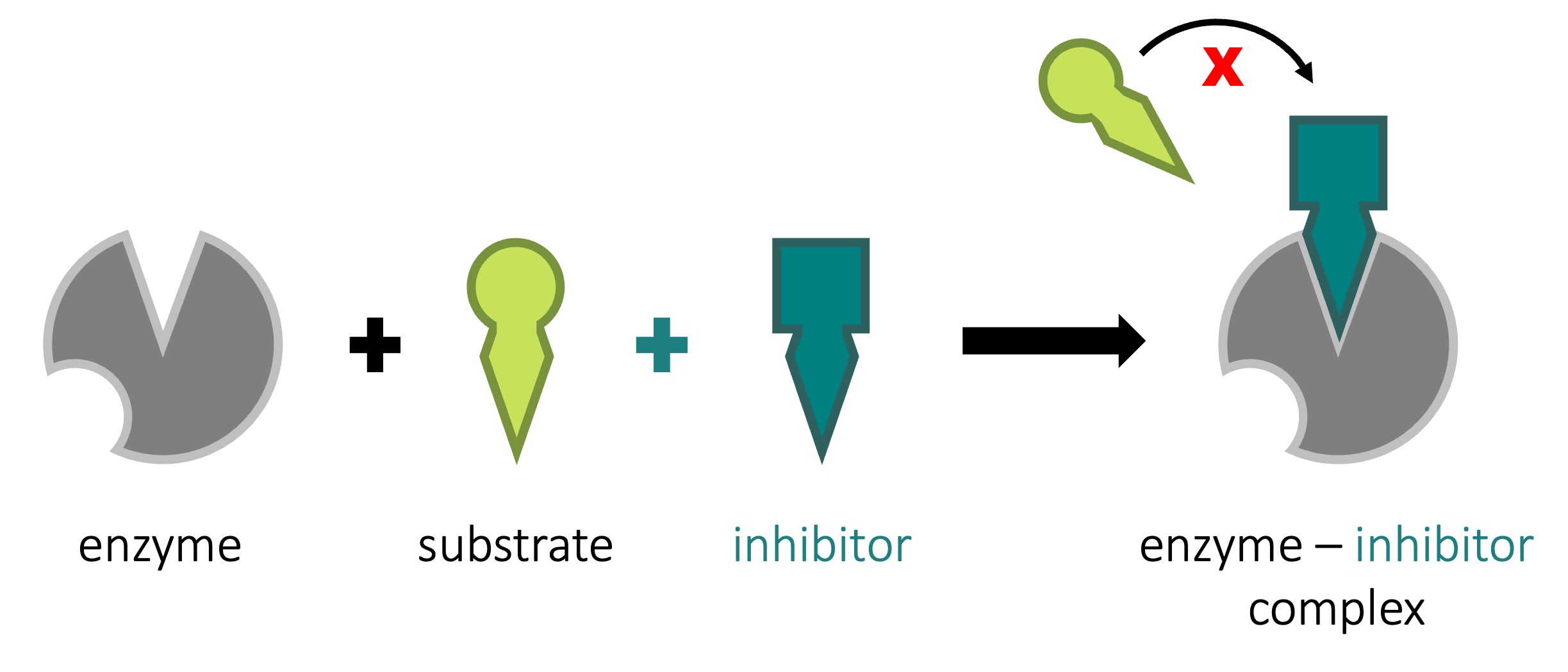

Non-Competitive Inhibition
Non-competitive inhibition involves a molecule binding to a site other than the active site (an allosteric site)
-
The binding of the inhibitor to the allosteric site causes a conformational change to the enzyme’s active site
-
As a result of this change, the active site and substrate no longer share specificity, meaning the substrate cannot bind
-
As the inhibitor is not in direct competition with the substrate, increasing substrate levels cannot mitigate the inhibitor’s effect
Cyanide is a poison which prevents aerobic respiration, leading to eventual death by non-competitive inhibition
-
Cyanide binds to an allosteric site on cytochrome oxidase – a carrier molecule that forms part of the electron transport chain
-
By changing the shape of the active site, cytochrome oxidase can no longer pass electrons to the final acceptor (oxygen)
-
Consequently, the electron transport chain cannot continue to function and ATP is not produced via aerobic respiration