Enzyme Activity
Various factors may affect the activity of enzymes, by either affecting the frequency of enzyme-substrate collisions or by affecting the capacity for the enzyme and substrate to interact (e.g. denaturation)
-
Temperature, pH and substrate concentration will all influence the rate of activity of an enzyme
Temperature
-
Low temperatures result in insufficient thermal energy for the activation of an enzyme-catalysed reaction to proceed
-
Increasing the temperature will increase the speed and motion of both enzyme and substrate, resulting in higher enzyme activity
-
This is because a higher kinetic energy will result in more frequent collisions between the enzymes and substrates
-
At an optimal temperature (may vary for different enzymes), the rate of enzyme activity will be at its peak
-
Higher temperatures will cause enzyme stability to decrease, as the thermal energy disrupts the enzyme’s hydrogen bonds
-
This causes the enzyme (particularly the active site) to lose its shape, resulting in the loss of activity (denaturation)

pH
-
Changing the pH will alter the charge of the enzyme, which in turn will alter protein solubility and overall shape
-
Changing the shape or charge of the active site will diminish its ability to bind the substrate, abrogating enzyme function
-
Enzymes have an optimal pH (may differ between enzymes) and moving outside this range diminishes enzyme activity

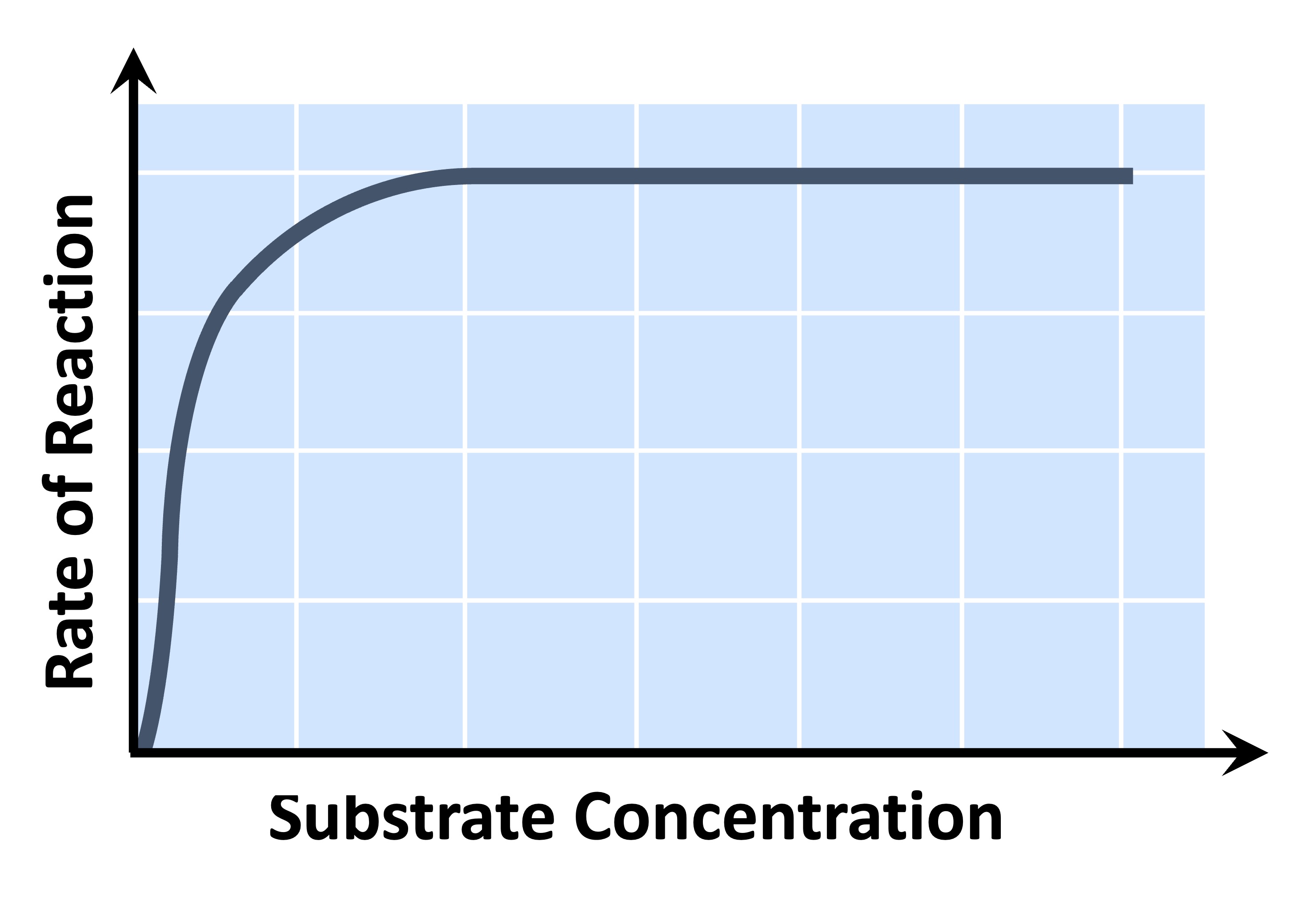
Substrate Concentration
-
Increasing substrate concentration will increase the activity of a corresponding enzyme
-
More substrates mean there is an increased chance of enzyme and substrate colliding and reacting within a given period
-
After a certain point, the rate of activity will cease to rise regardless of any further increases in substrate levels
-
This is because the environment is saturated with substrate and all enzymes are bound and reacting (Vmax)