Enzymes
An enzyme is a globular protein which acts as a biological catalyst by speeding up the rate of a chemical reaction
-
Enzymes are not changed or consumed by the reactions they catalyse and can be reused (hence are only required in low amounts)
Enzymes are typically named after the molecules they react with (called the substrate) and end with the suffix ‘-ase’
-
For example, lipids are broken down by the enzyme lipase while proteins are digested by proteases
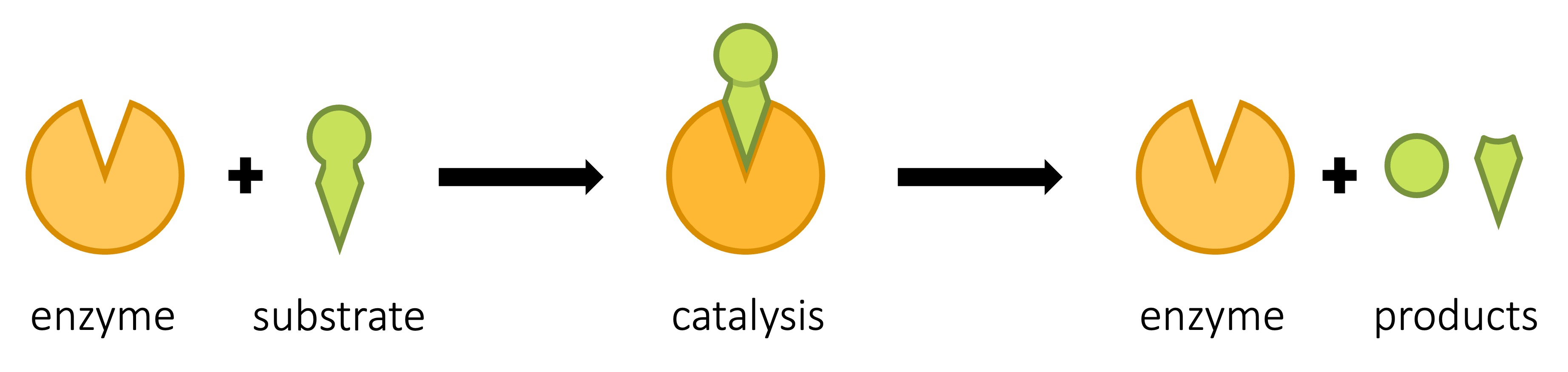
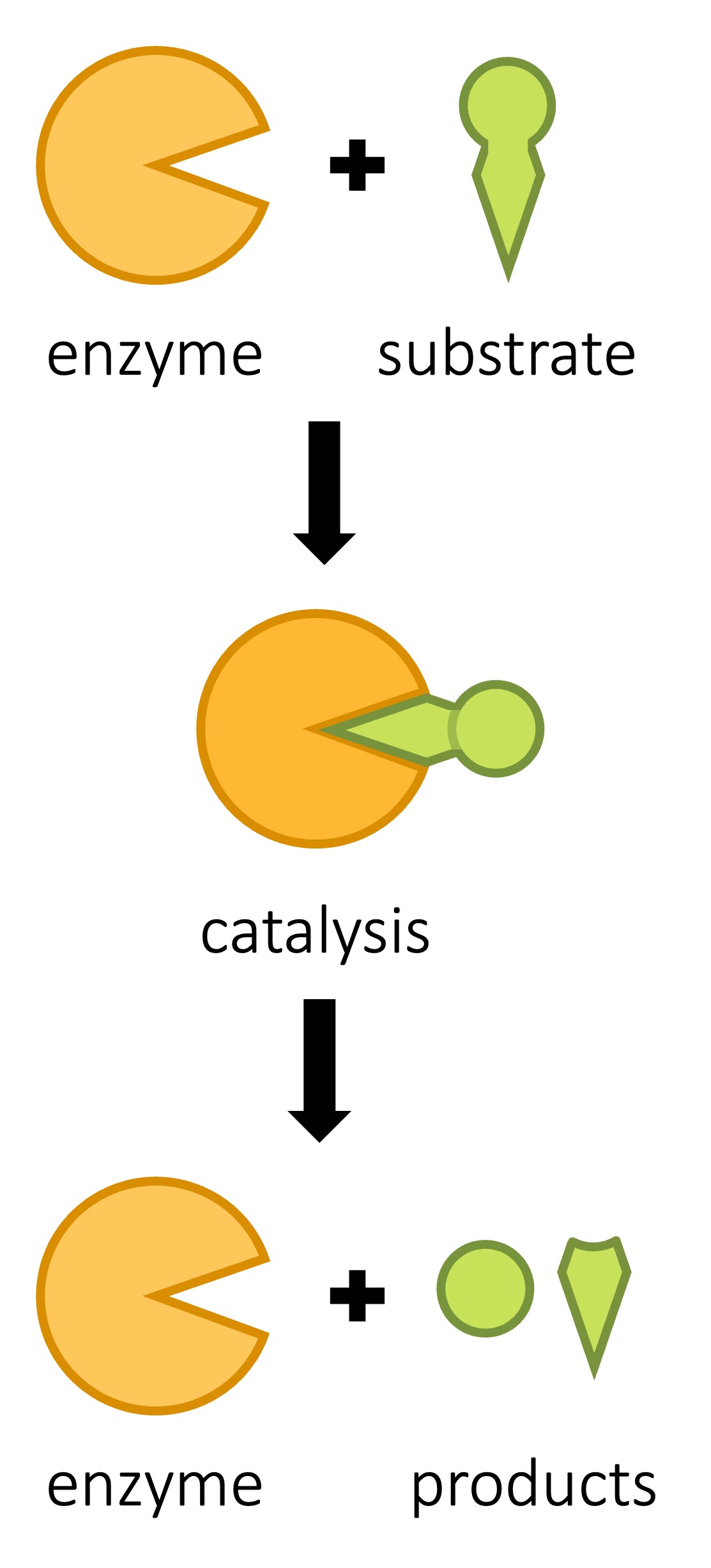
The region on the surface of the enzyme to which a substrate molecule binds is called the active site
-
The active site is composed of a few amino acids only, but interactions between these amino acids ensures that the overall shape and chemical properties of the active site complement the substrate


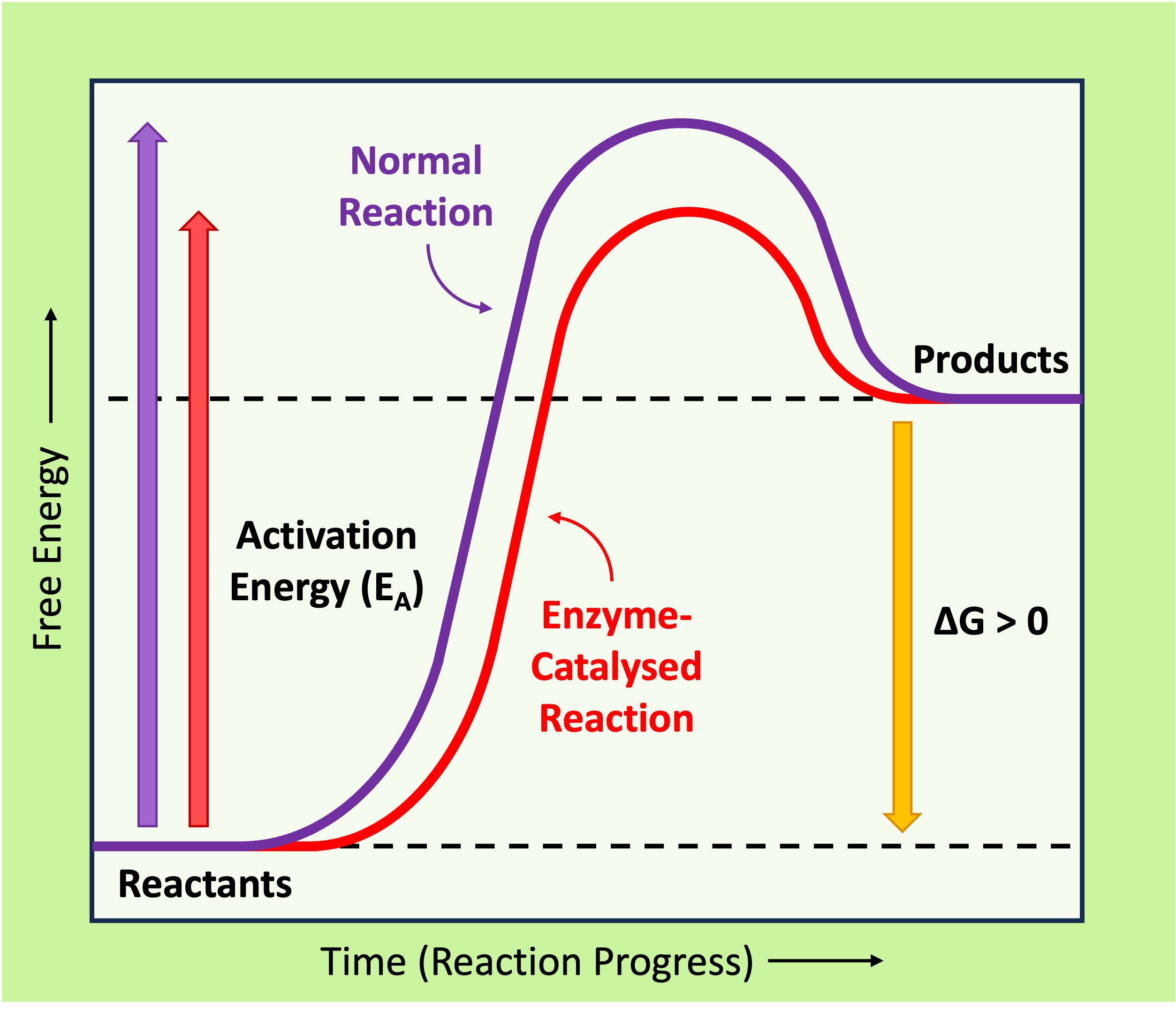
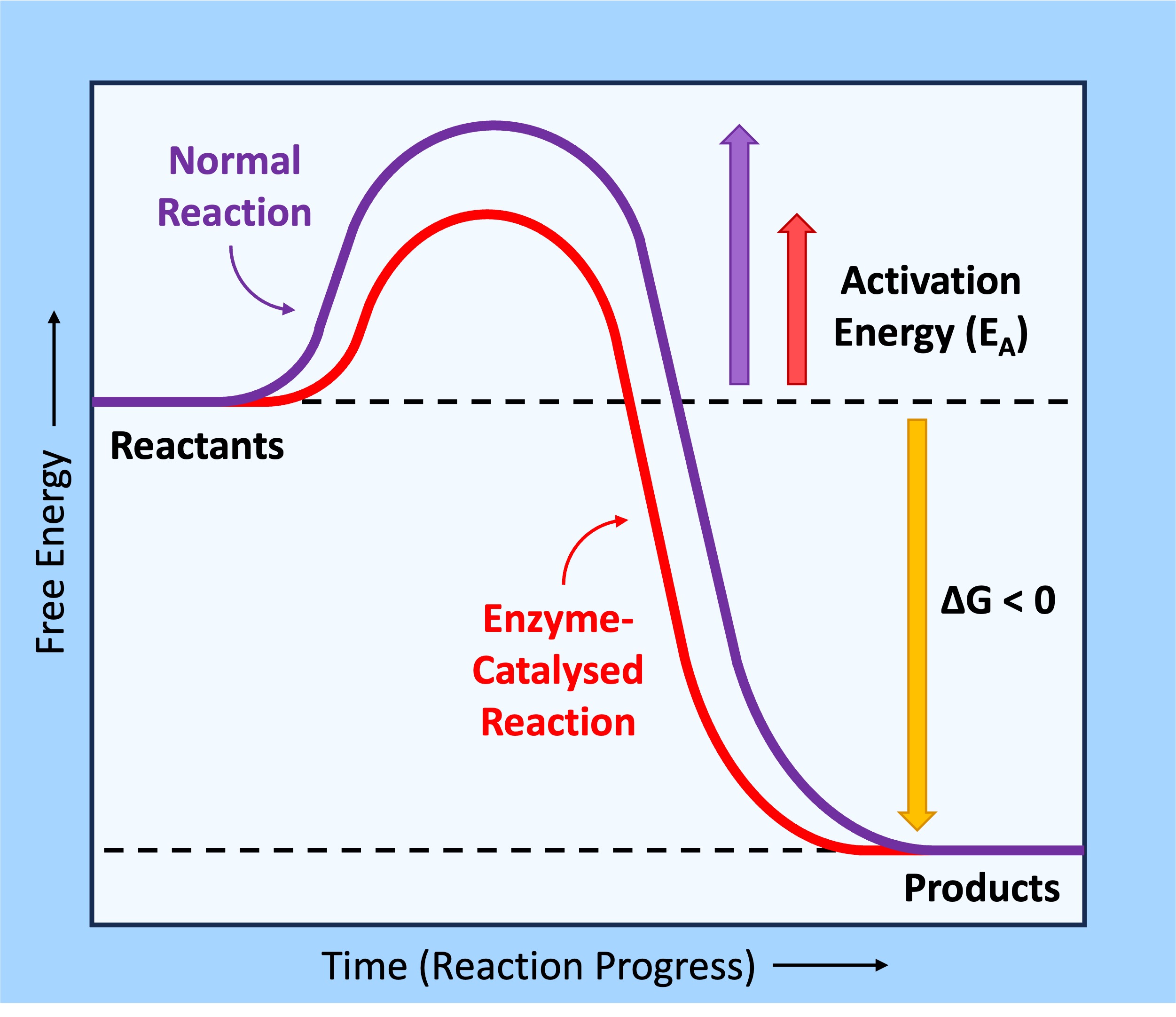
Activation Energy
Every chemical reaction requires a certain amount of energy in order to proceed – this is the activation energy (EA)
-
Enzymes speed up the rate of a biochemical reaction by lowering the activation energy, meaning less energy is needed to convert the substrate into a product and the reaction proceeds at a faster rate
If the reactants contain more energy than the products, the free energy is released into the system (exergonic)
-
These reactions are usually catabolic (breaking down), as energy is released from broken bonds within a molecule
If the reactants contain less energy than the products, free energy is lost to the system (endergonic)
-
These reactions are usually anabolic (building up), as energy is required to synthesise bonds between molecules
Exergonic Reaction