Energy Flow
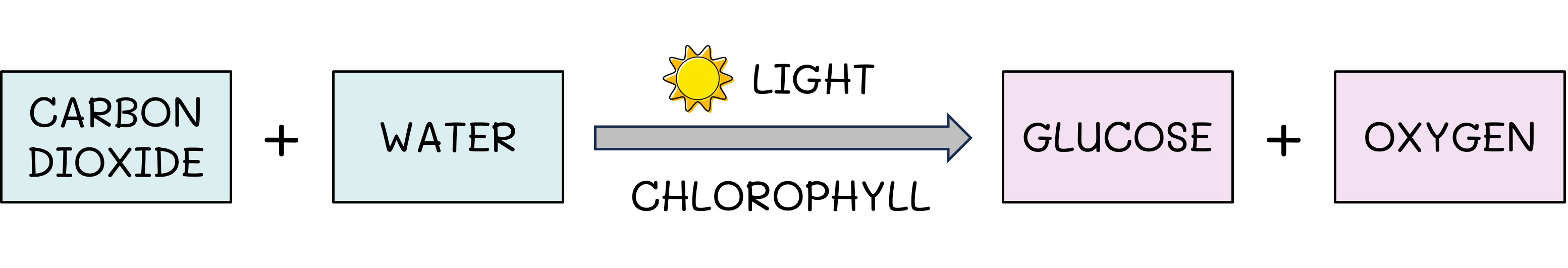
Photosynthesis
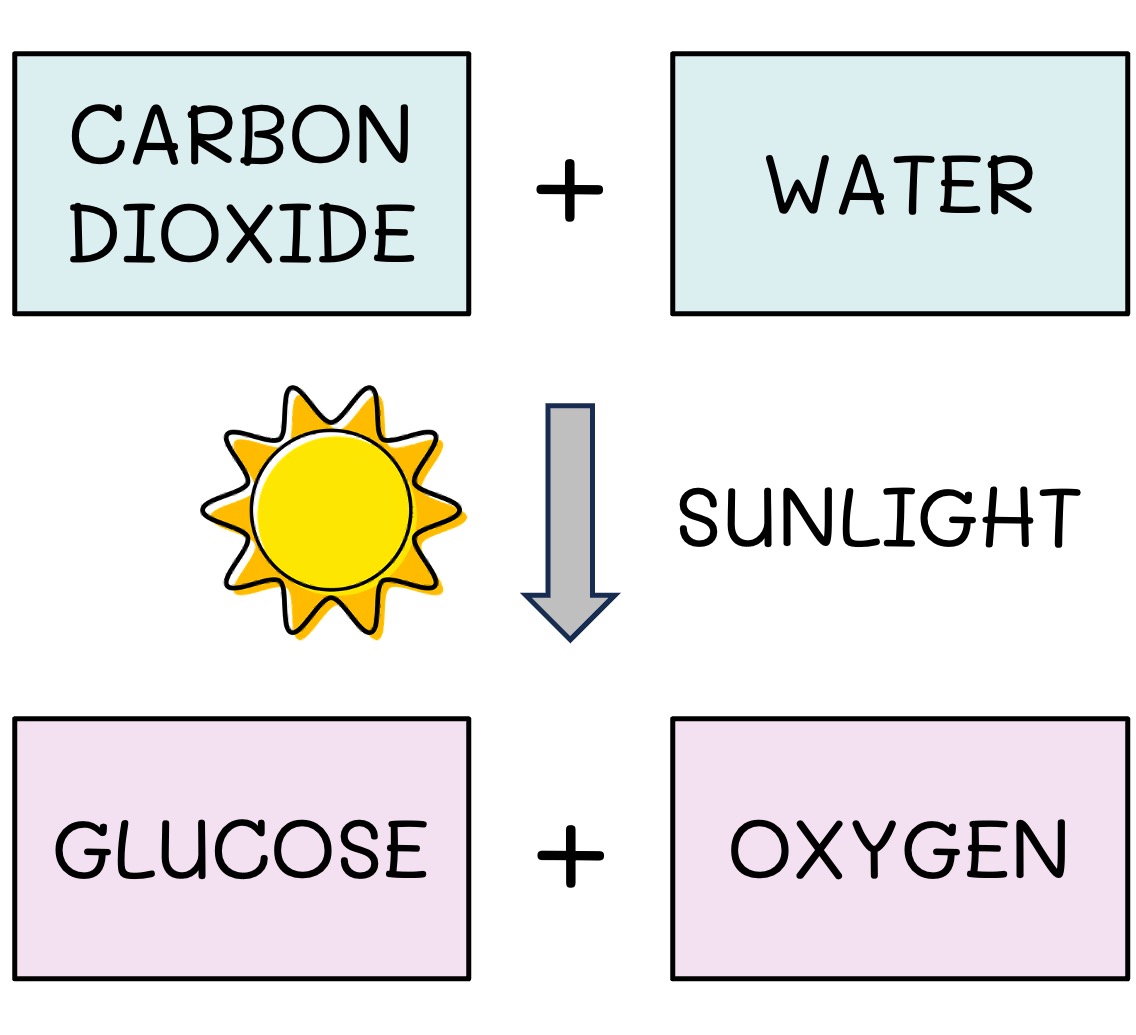
The initial source of energy for almost all communities is sunlight
-
Light is absorbed and converted into usable chemical energy (ATP) via the process of photosynthesis
-
This energy is used to make organic compounds (e.g. glucose) from inorganic molecules (CO2 and H2O)
-
The organic compounds can be used as building blocks to create a range of macromolecules needed by the cells and tissues
-
All green plants are photosynthetic, along with certain algae (e.g. seaweeds) and prokaryotes (e.g. cyanobacteria)
Photosynthesis Equation
Chemosynthesis
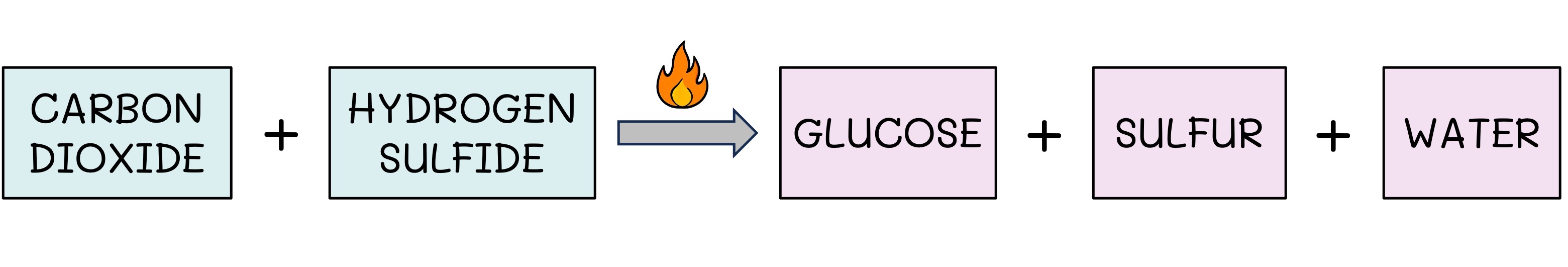
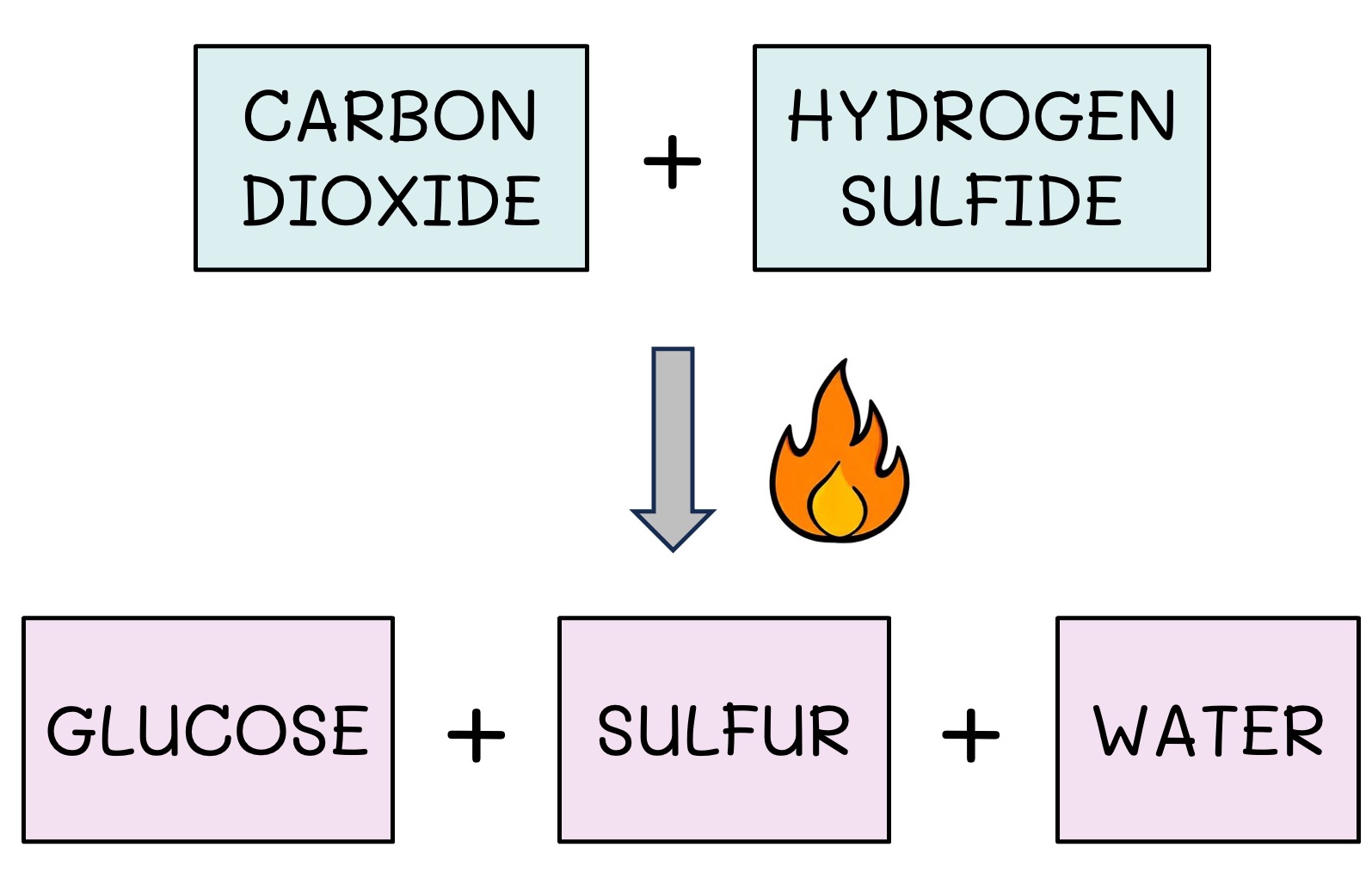
Some communities can derive energy from oxidation reactions involving inorganic compounds
-
These inorganic sources may include ammonia, hydrogen gas, hydrogen sulfide or iron oxide
-
Such communities may be located near deep sea hydrothermal vents or in underground caves where no light can penetrate
-
The specific chemical processes deployed by these organisms may vary depending on the inorganic chemical used as an energy source
-
Iron-oxidising bacteria found in the soil are an example of a chemoautotroph – they use the electrons from iron (oxidation) to produce ATP
Chemosynthesis Example
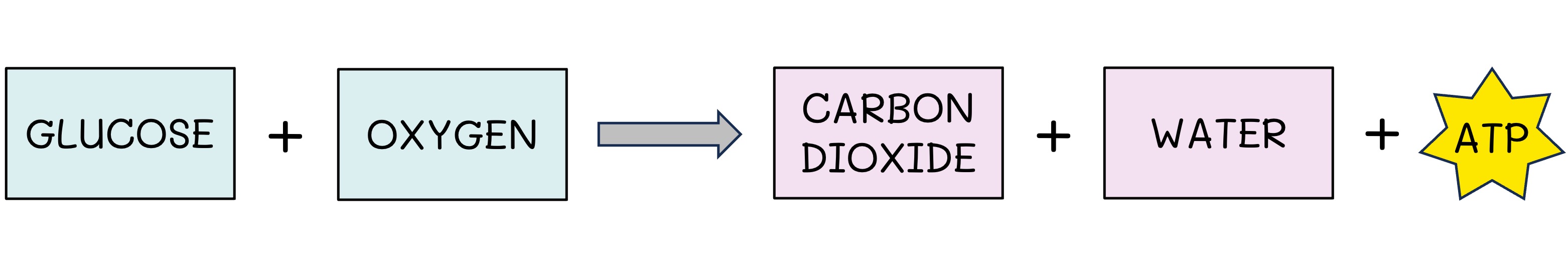
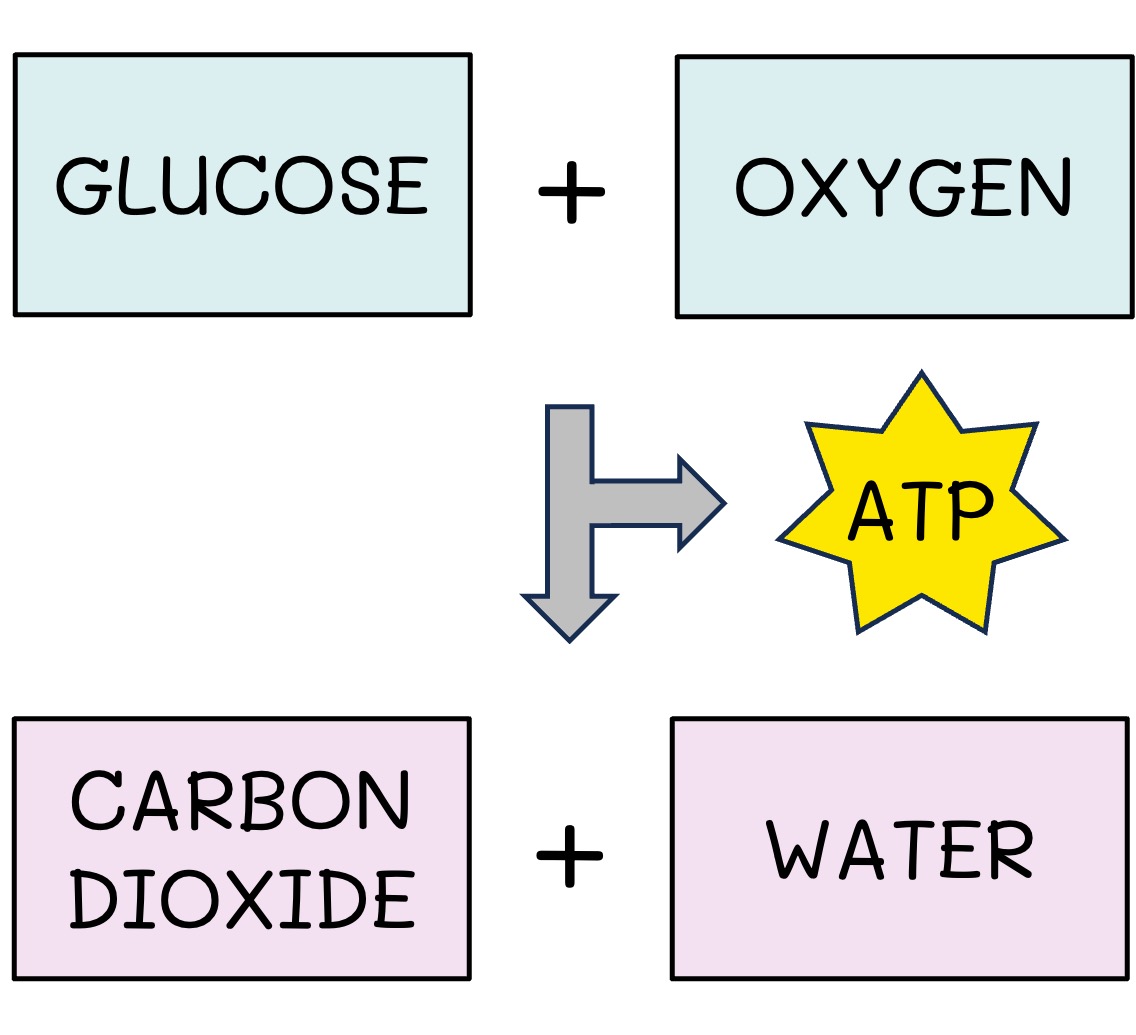
Cell Respiration
Organisms undertake cell respiration to release the chemical energy stored in organic compounds
-
Carbohydrates (glucose) serve as the primary fuel source, but other compounds (lipids, proteins) may be used
-
The organic compounds can either be produced by the organism itself (autotrophy) or obtained from other organisms (heterotrophy)
-
Cell respiration can occur anaerobically (no oxygen = low ATP yield) or aerobically (oxygen = high ATP yield)
-
The release of energy involves oxidation reactions (high energy electrons are used in the production of ATP)
Cell Respiration Equation