End Products
The Calvin cycle is responsible for the fixation of carbon from inorganic sources to form the different types of organic compounds required by the cell
-
The four main types of carbon compounds present in a cell are carbohydrates, lipids, proteins and nucleic acids
Synthesis of organic molecules happens via a variety of metabolic reactions that take place within the cytoplasm
-
The fraction of triose phosphates that are not used to regenerate RUBP can be converted in the cytosol to form vital biomacromolecules
Some organic compounds require the additional assimilation of mineral nutrients
-
Proteins require the incorporation of nitrogen (and potentially sulphur) to form amino acids
-
Nucleic acids require the incorporation of phosphorus (phosphate group) and nitrogen (base) to form nucleotides
-
Certain types of lipids may also require additional mineral nutrients (e.g. phospholipids need phosphorus)
Organic Synthesis
Carbohydrate
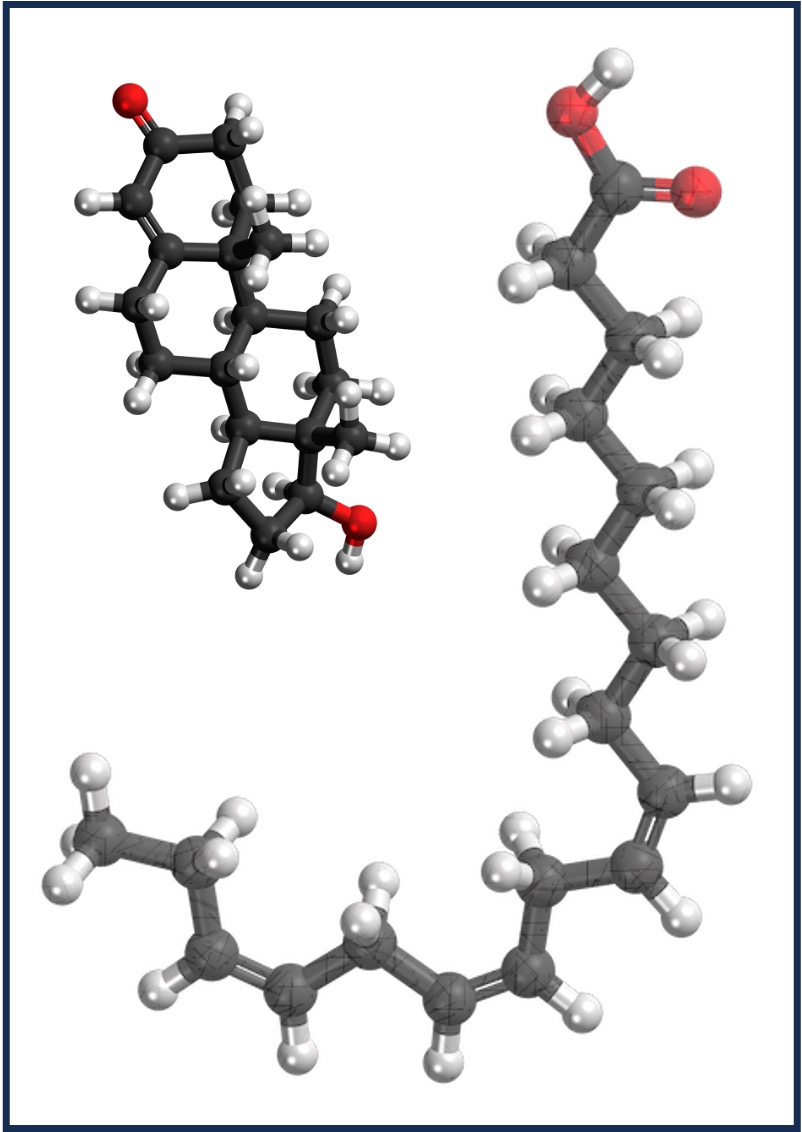
Lipids
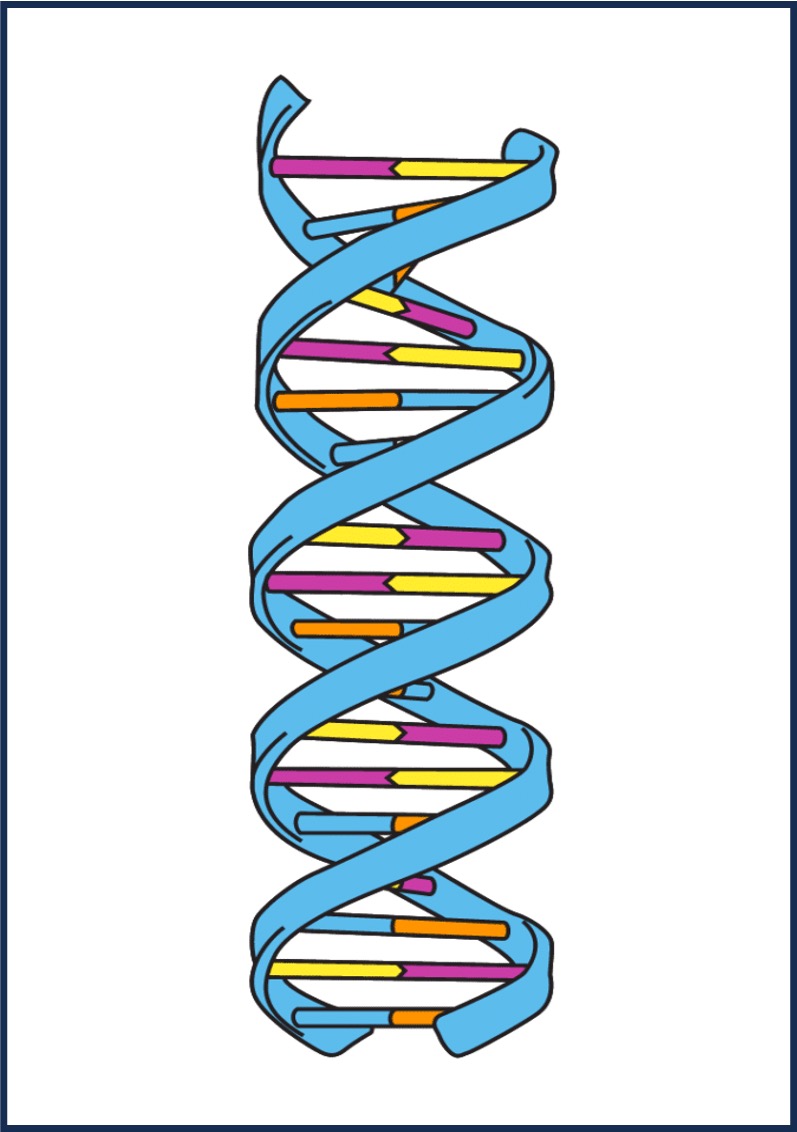
Nucleic Acid
Protein
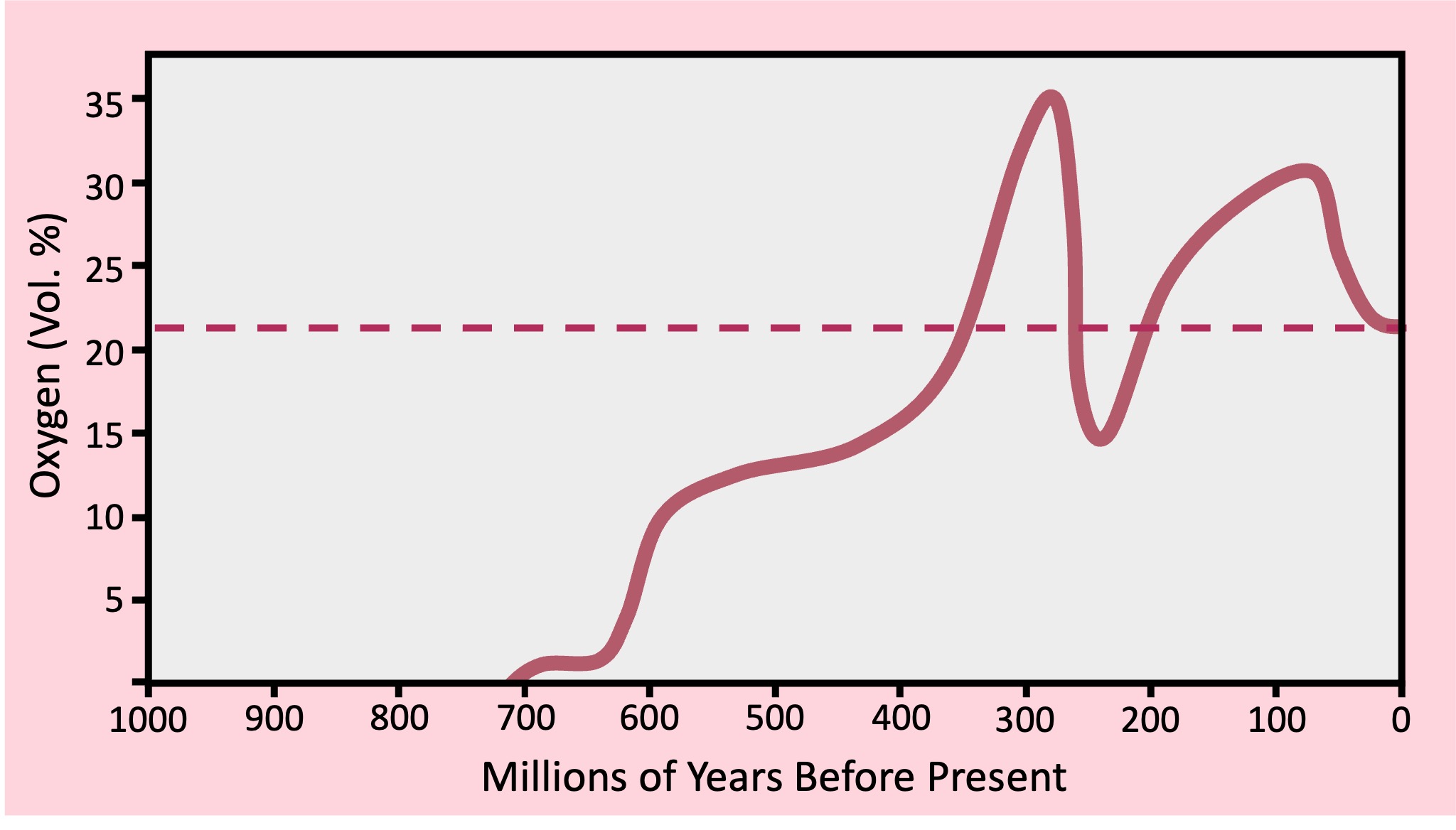
The photolysis of water generates protons and electrons that are used in the light dependent reactions, but oxygen is a waste product is this process
-
Photosynthesis generates the only significant source of oxygen gas in the known universe
Before the evolution of photosynthetic organisms, any free oxygen produced on Earth was chemically captured and stored
-
As photosynthetic organisms began to saturate the environment with oxygen, the Earth’s atmosphere, oceans and geological processes began to change
Earth’s oceans initially had high levels of dissolved iron (released from the crust by underwater volcanic vents)
-
When iron reacts with oxygen gas it undergoes a chemical reaction to form an insoluble precipitate (iron oxide)
-
The build up of iron precipitates created oceanic deposits called banded iron formations (BIFs)
-
When the iron in the ocean was completely consumed, oxygen gas started accumulating in the atmosphere
Free oxygen is toxic to obligate anaerobes and an increase in atmospheric oxygen levels may have wiped out many of these species
-
The rise in atmospheric oxygen levels was a critical determinant to the evolution of aerobically respiring organisms
-
The oxygenation of the atmosphere also led to the development of an ozone layer which limited exposure to harmful radiation
Oxygenation of Earth

Banded Iron Formations (BIFs)