Electron Transport Chain
The final stage of aerobic respiration is the electron transport chain, which is located on the inner mitochondrial membrane
-
The inner membrane is arranged into folds (cristae), which increases the surface area available for the transport chain
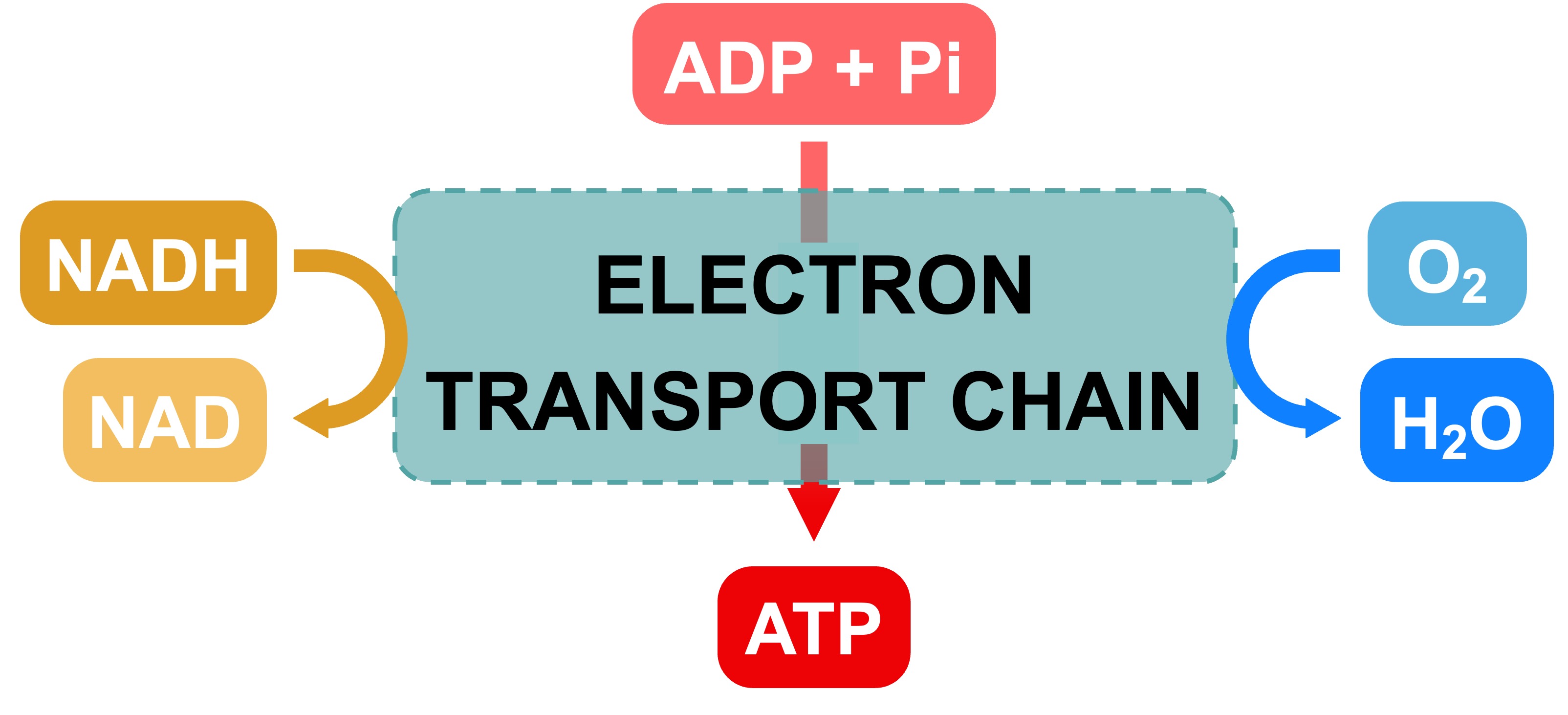
The electron transport chain releases the energy stored within the reduced hydrogen carriers in order to synthesise ATP
-
This is called oxidative phosphorylation, as the energy to synthesise ATP is derived from the oxidation of hydrogen carriers
Oxidative phosphorylation occurs over a number of distinct steps:
-
Proton pumps create an electrochemical gradient (proton motive force)
-
ATP synthase uses the subsequent diffusion of protons (chemiosmosis) to synthesise ATP
-
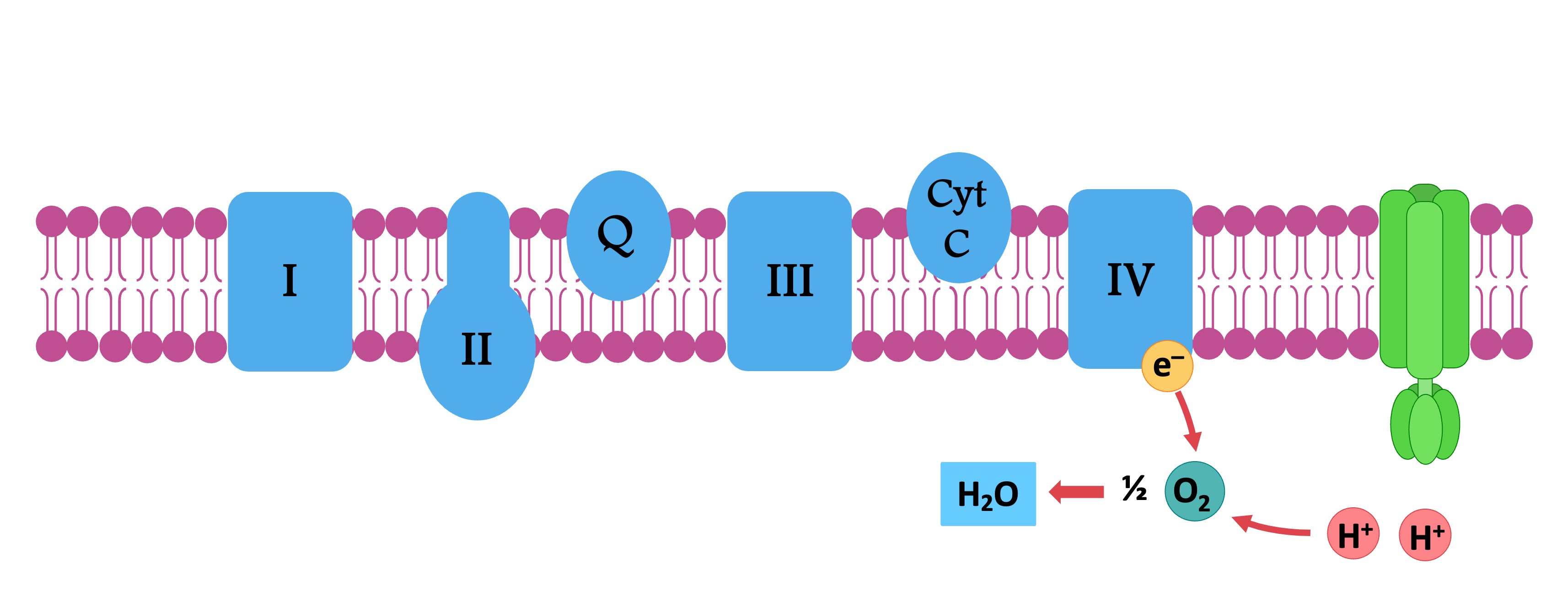
Oxygen accepts electrons and protons to form water
Oxidative Phosphorylation
Step 1: Proton Motive Force
-
The hydrogen carriers (NADH and FADH2) are oxidised and release high energy electrons and protons
-
The electrons are transferred to the electron transport chain, which consists of several transmembrane carrier proteins
-
As electrons pass through the chain, they lose energy – which is used by the chain to pump protons (H+ ions) from the matrix
-
The accumulation of H+ ions within the intermembrane space creates an electrochemical gradient (or a proton motive force)
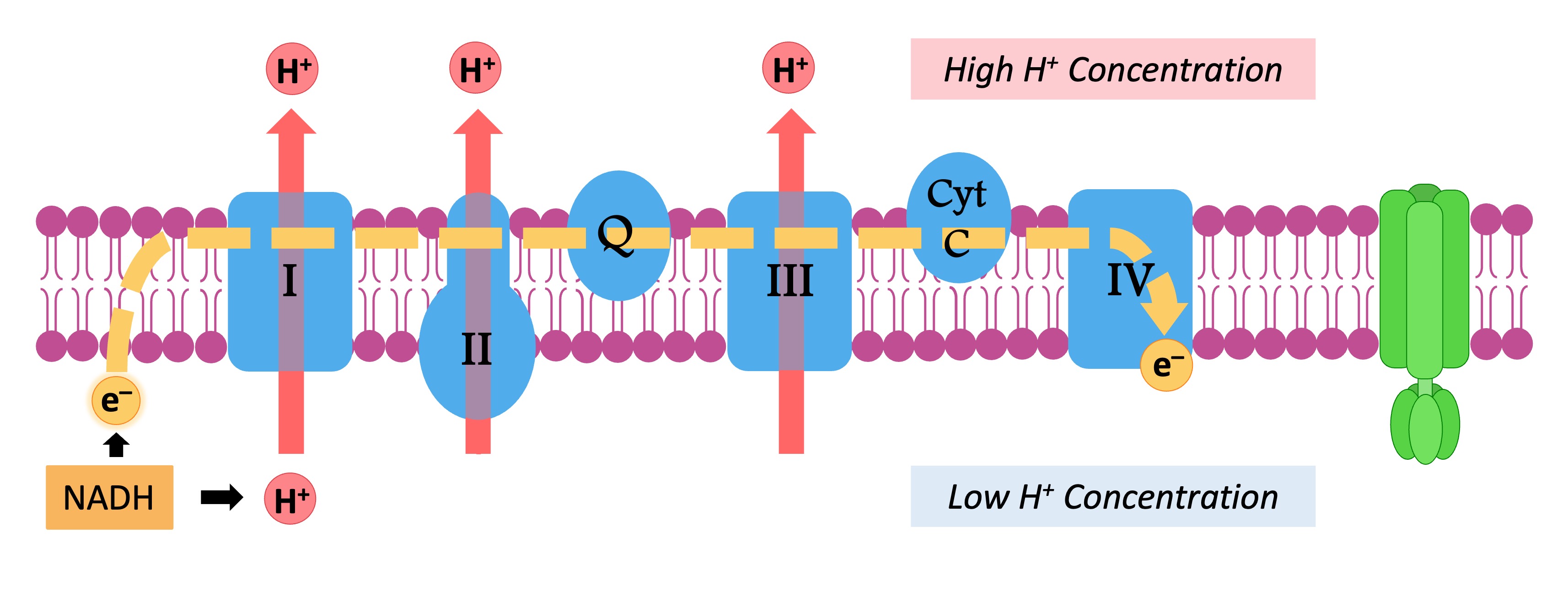
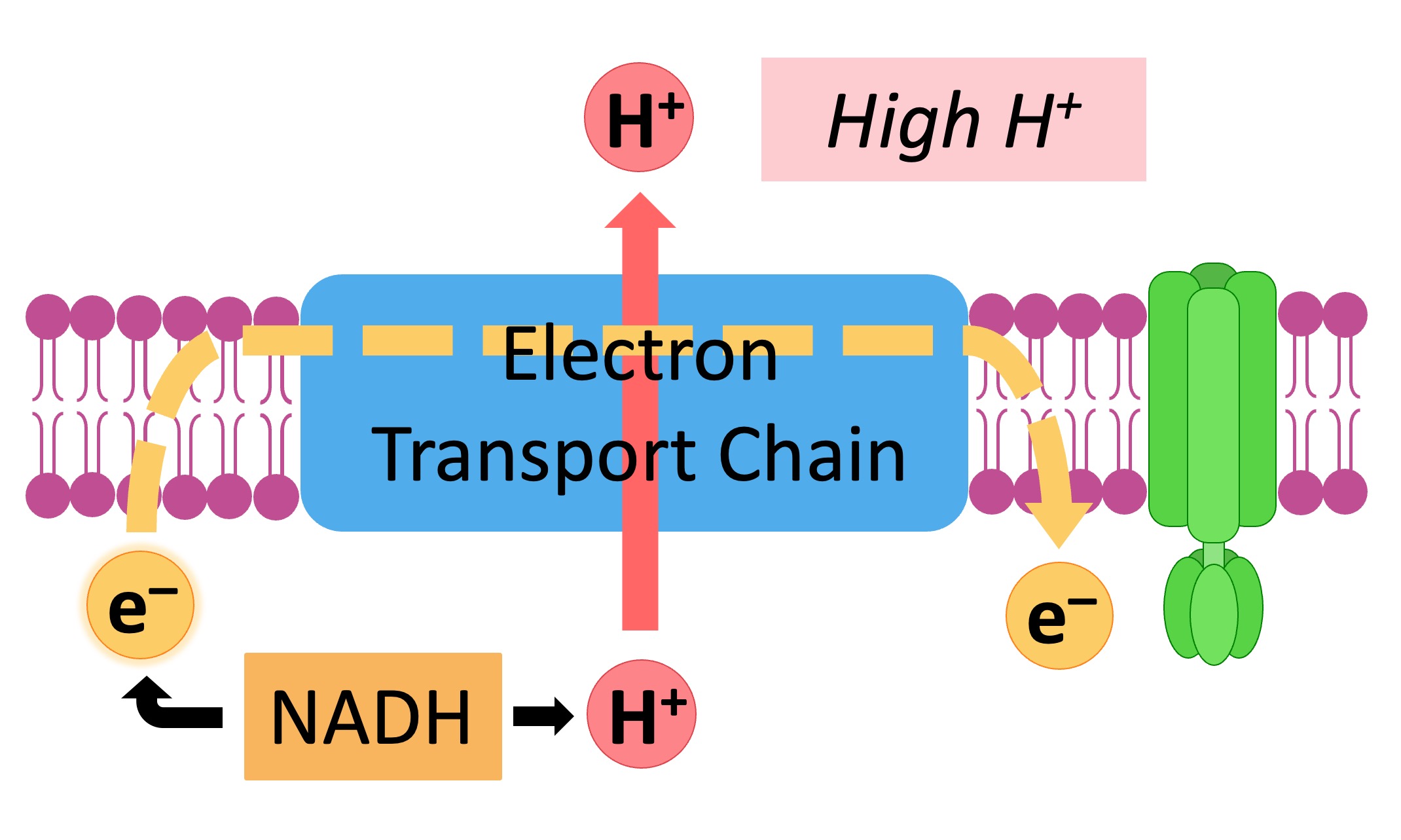
High energy electrons are shuttled through the transport chain
The released energy translocates protons, creating a gradient
Step 2: Chemiosmosis
-
The proton motive force will cause H+ ions to move down their electrochemical gradient and diffuse back into matrix
-
This diffusion of protons is called chemiosmosis and is facilitated by the transmembrane enzyme ATP synthase
-
As the H+ ions move through ATP synthase they trigger the molecular rotation of the enzyme, synthesising ATP
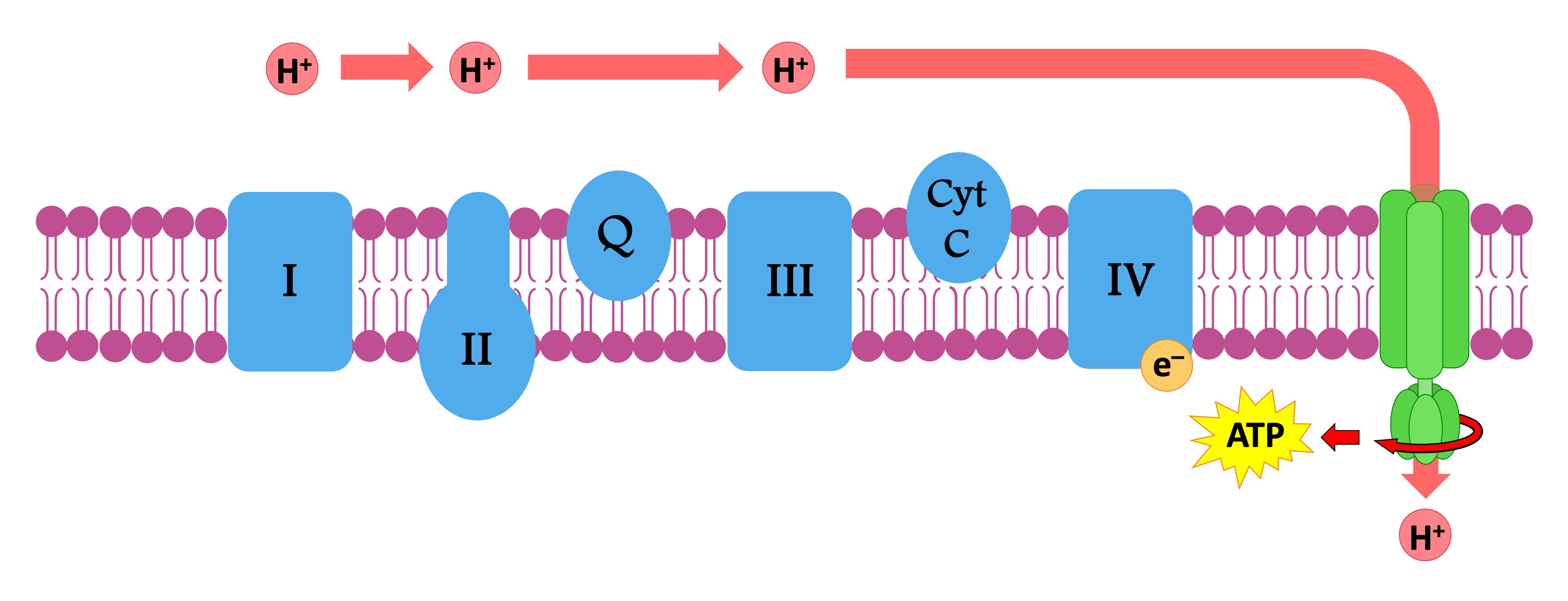

Protons are transported down their gradient (chemiosmosis)
ATP synthase uses this proton flow to synthesise ATP
Step 3: Reduction of Oxygen
-
In order for the electron transport chain to continue functioning, the de-energised electrons must be removed
-
Oxygen acts as the final electron acceptor, removing the de-energised electrons to prevent the chain from becoming blocked
-
Oxygen also binds with free protons in the matrix to form water – removing matrix protons maintains the hydrogen gradient
-
In the absence of oxygen, hydrogen carriers cannot transfer energised electrons to the chain and ATP production is halted