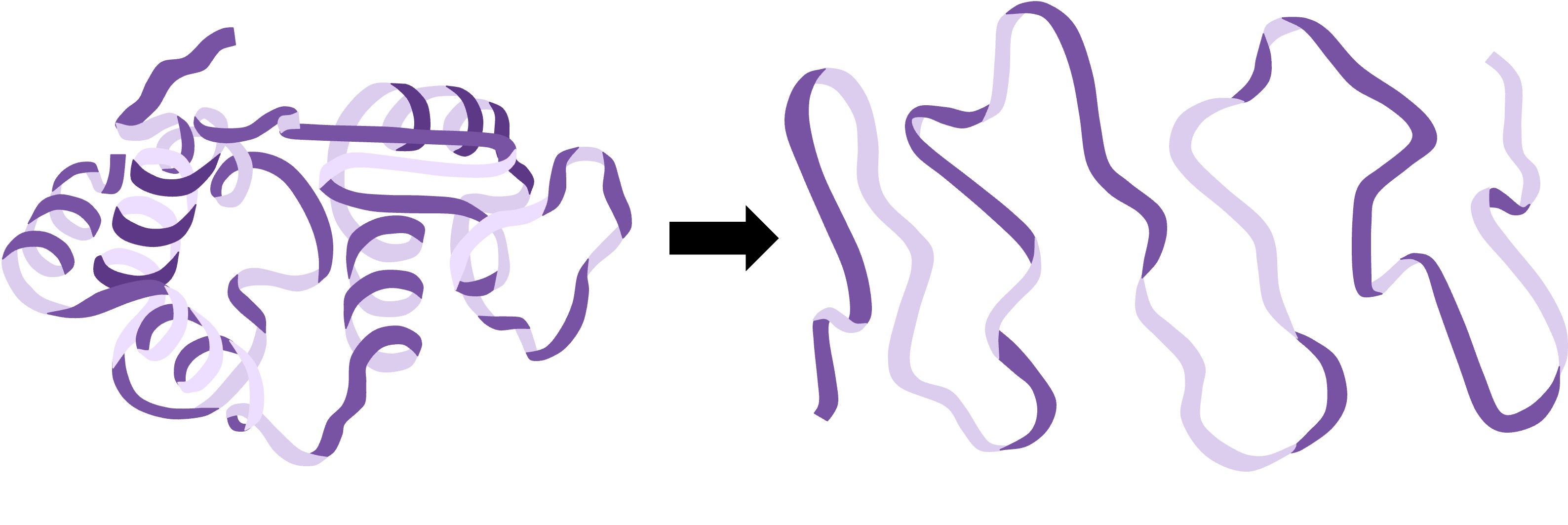
Denaturation
Denaturation is a structural change in a protein that results in the loss (usually permanent) of its biological properties
-
Because the way a protein folds determines its function, any change or abrogation of the three dimensional structure will alter its activity
Denaturation of proteins can usually be caused by two key conditions – heat (high temperatures) and pH

Temperature:
-
High levels of thermal energy may disrupt the hydrogen bonds that hold the protein together
-
As these bonds are broken, the protein will begin to unfold and lose its capacity to function as intended
-
Temperatures at which proteins denature may vary, but most human proteins function optimally at body temperature (~37ºC)
pH:
-
Amino acids are zwitterions, neutral molecules possessing both negatively (COO–) and positively (NH3+) charged regions
-
Changing the pH will alter the charge of the protein, which in turn will alter protein solubility and overall shape
-
All proteins have an optimal pH which is dependent on the environment in which it functions (e.g. stomach proteins require an acidic environment to operate, whereas blood proteins function best at a neutral pH)
Effect of pH on Proteins