Cohesive Properties
Cohesion
Water is cohesive – a water molecule is able to stick to other water molecules via the formation of hydrogen bonds
-
The hydrogen bonding between water molecules allows the liquid to resist low levels of external force (it creates surface tension)
-
The high surface tension of water makes it sufficiently dense for certain smaller organisms to move along its surface
Water striders are small insects that can move along the surface of water due to its high surface tension
-
Water striders distribute their weight via long legs that contain thousands of microscopic hairs to trap air and increase buoyancy
-
The high surface tension of water makes it sufficiently dense for certain smaller organisms to move along its surface
Surface Tension
Adhesion
Water is also adhesive – a water molecule is able to stick to other polar or charged molecules via the formation of polar associations
-
Attraction to charged or polar surfaces (such as cellulose cell walls) allows water to flow in opposition to gravitational forces (capillary action)
-
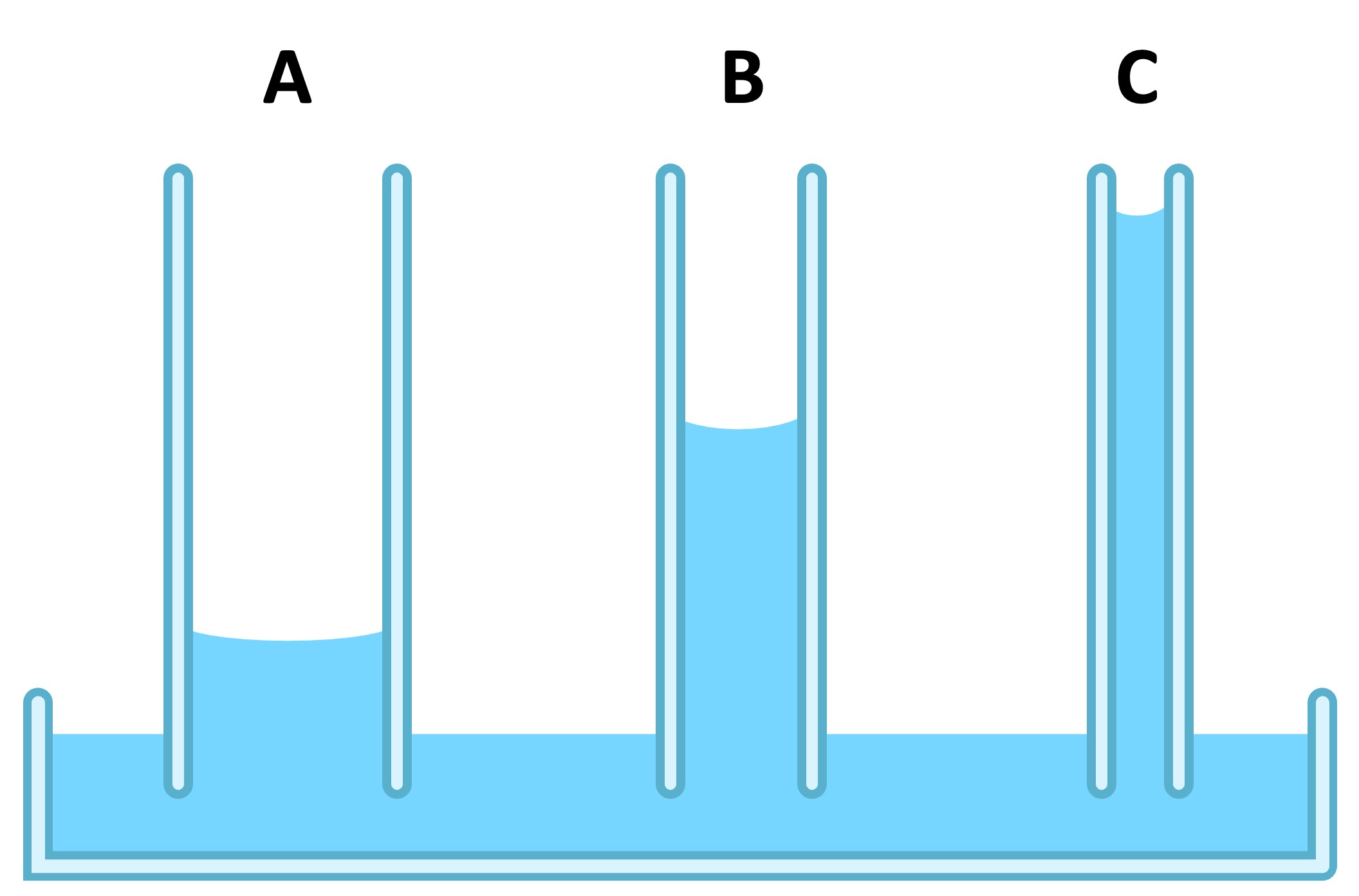
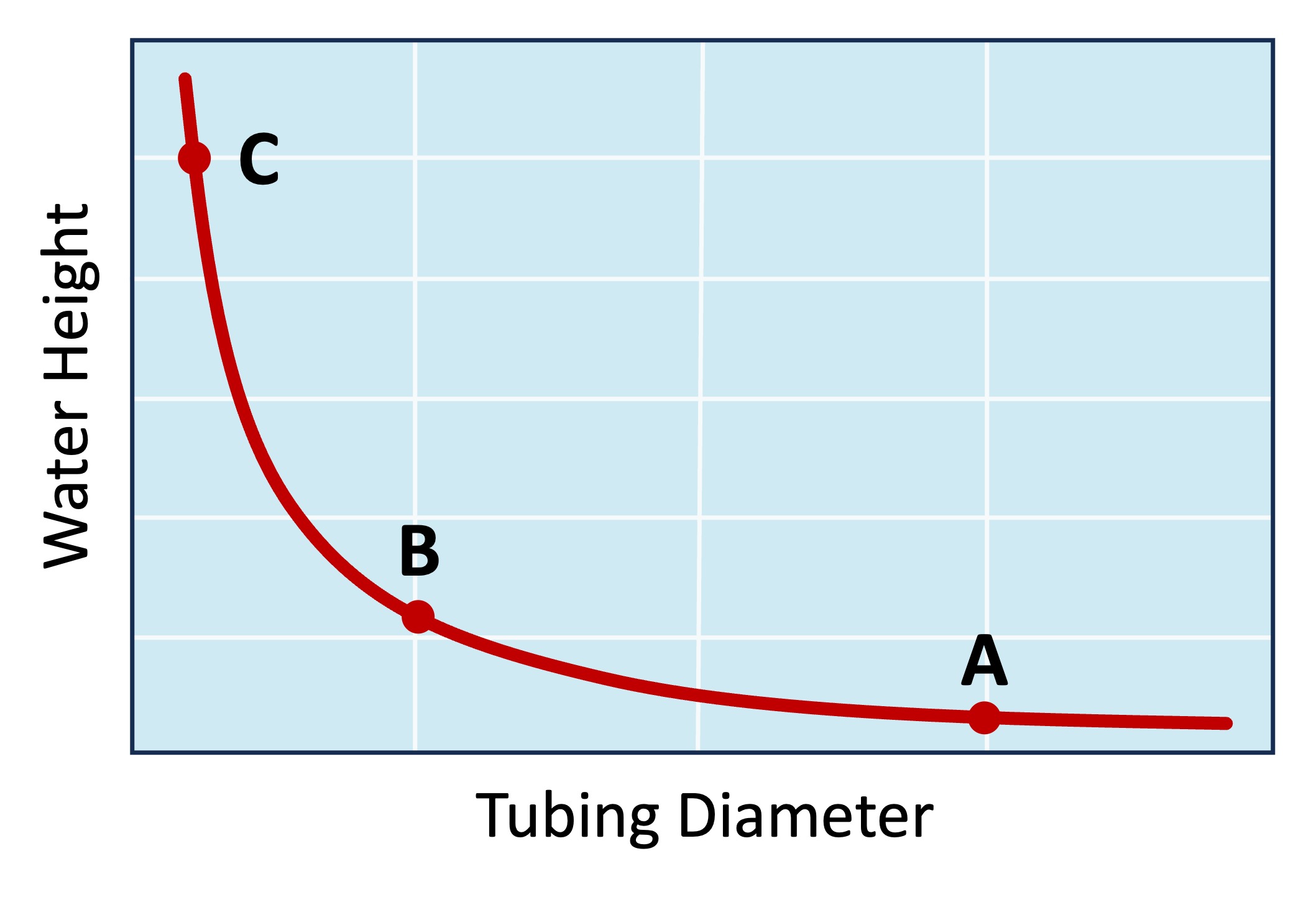
The strength of the capillary action will be dependent on the diameter of the pore through which the water moves (smaller diameter = more action)
Capillary action is necessary to allow water to be transported up plant stems via a transpiration stream
-
The loss of water vapour from the leaves (via evaporation) and the absorption of water into the roots (via osmosis) creates a pressure gradient
-
Water will move along this gradient by using capillary action and cohesion to be transported up the stem of the plant via narrow xylem vessels
Capillary action is also responsible for the movement of water through the soil from the deeper water table
-
The type of soil will influence the strength of the capillary action, affecting the efficacy of agricultural practices
Capillary Action