Catalysis
A catalyst is a substance that allows a reaction to proceed at a faster rate or under different conditions tha otherwise possible
-
Enzymes are biological catalysts that are not consumed by the specific reactions they catalyse (so can operate in low amounts)
Enzymes allow chemical reactions to proceed within a biologically relevant passage of time
-
Without enzymes, DNA replication would be unable to occur within the lifetime of a cell (preventing organismal growth and repair)
-
Without enzymes, food would be unable to be chemically digested within the period of transit through the digestive tract (meaning insufficient nutrient absorption)
Enzymes also allow for chemical reactions to proceed at biologically appropriate temperatures
-
Without enzymes, chemical reactions would require higher temperatures which could denature cell components (homeostasis would not be maintained)
Enzyme-Substrate Interactions
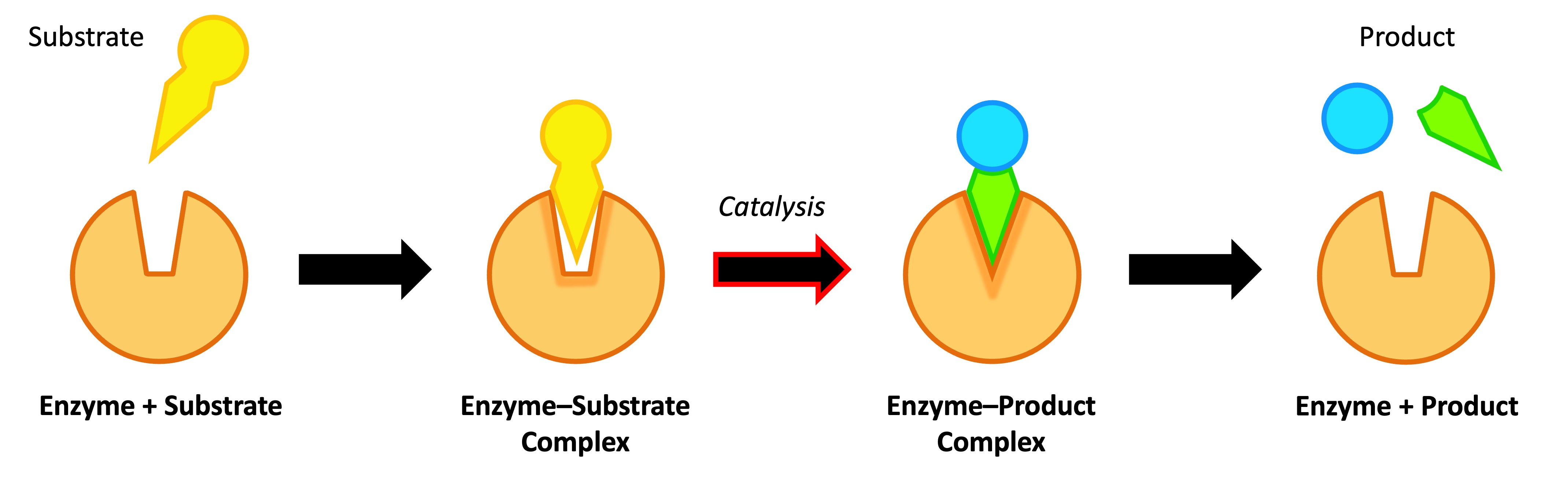
Enzyme catalysis requires that the substrate be brought into close physical proximity with the active site
-
When a substrate binds to the enzyme’s active site, an enzyme-substrate complex is formed
-
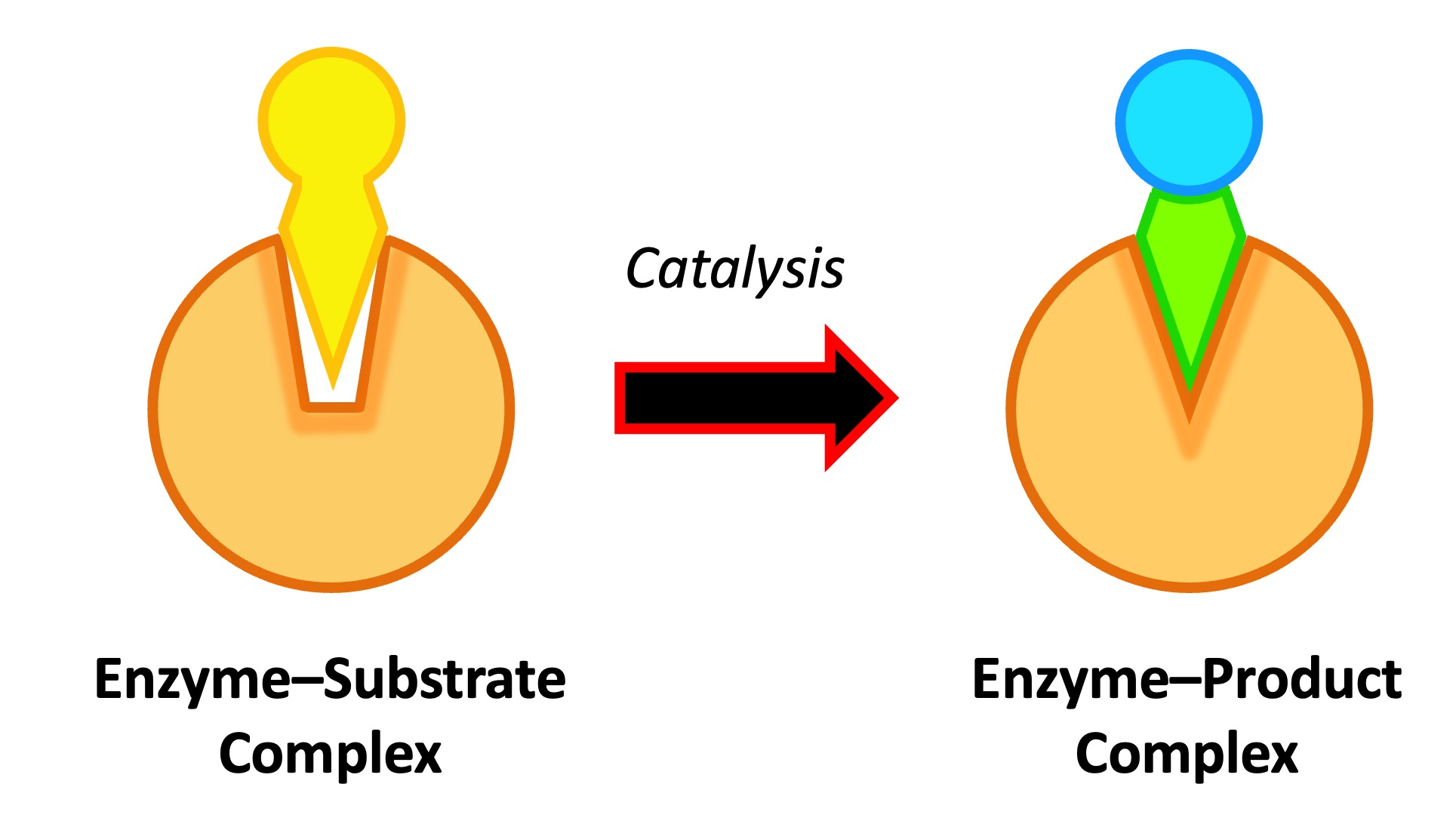
The enzyme catalyses the conversion of the substrate into product, creating an enzyme-product complex
-
The enzyme and product then dissociate – as the enzyme was not consumed, it can continue to catalyse further reactions
According to the induced fit model, the enzyme’s active site is not a completely rigid fit for the substrate
-
Instead, the active site will undergo a conformational change when exposed to a substrate to improve binding
-
This explains how enzymes may exhibit broad specificity (e.g. lipase can bind to a variety of lipids)
-
It also explains how catalysis may occur (the conformational change stresses bonds in the substrate, increasing reactivity)
-
Induced Fit Binding
Collision Theory
Enzyme reactions occur in aqueous solutions (cytoplasm, interstitial fluid), with the substrate and enzyme moving randomly (Brownian motion)
-
Sometimes an enzyme may be fixed in position (e.g. membrane-bound) – this serves to localise reactions to particular sites
For an enzymatic reaction to occur, the substrate and enzyme must physically collide in the correct orientation to facilitate binding to the active site
-
The rate of enzyme catalysis can be increased by improving the frequency of collisions in two ways:
-
Increasing the molecular motion of the particles (thermal energy can be introduced to increase kinetic energy)
-
Increasing the concentration of particles (either substrate or enzyme concentrations)
-