

Aquatic Impacts
Global warming is changing the environmental conditions of aquatic habitats, including water temperature, pH and the distribution of ocean currents
-
These oceanic changes can potentially change the distribution and interactions between species within marine communities
Ocean Currents
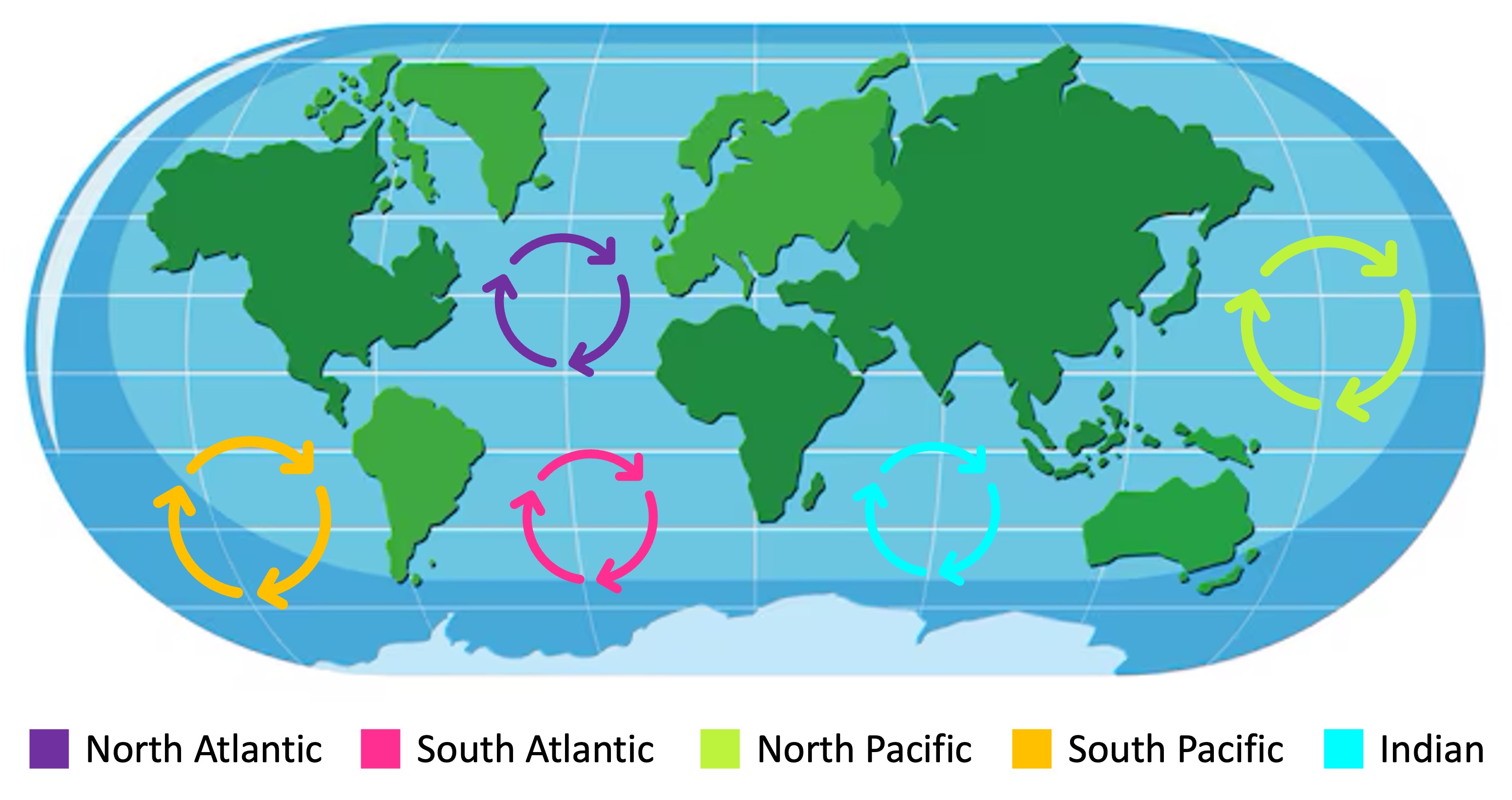
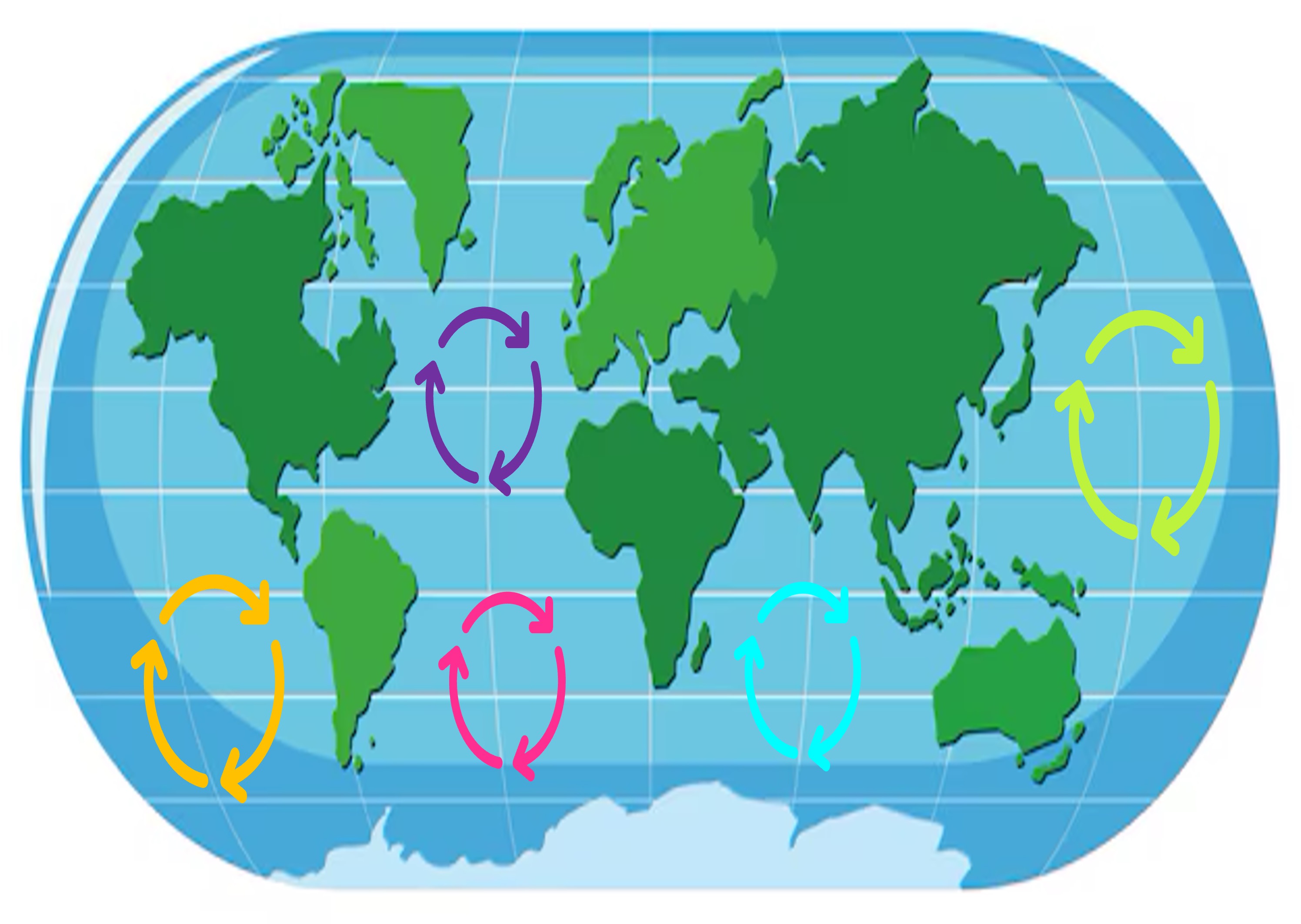
An ocean current is the continuous, directed movement of seawater driven by abiotic conditions such as temperature, wind and salinity
-
Surface ocean currents may collectively form large, permanent systems known as ocean gyres
Ocean currents play an important role in transferring heat and nutrients and changes in ocean currents can alter the timing and extent of these redistributions
-
Upwelling occurs when colder, nutrient-rich water rises to the surface as a consequence of wind and wave movement displacing the warmer surface water
-
Climate change is causing ocean temperatures to rise, which is preventing nutrient upwelling and thereby decreasing ocean primary production
-
The reduction in primary production is consequently impacting the flow of energy through marine food chains, compromising ecosystem stability
Ocean Gyres
Coral Reefs
Climate change is also affecting the distribution and survival of the ocean’s coral reefs – which threatens to collapse entire marine ecosystems
-
The coral polyps that comprise the reef form a mutualistic relationship with a photosynthetic algae (zooxanthellae)
-
The zooxanthellae provide the polyps with a food source (via photosynthesis), while the polyps provide the algae with resources and protection
Ocean temperatures are increasing, which is negatively impacting the survival of the coral polyps and causing reefs to become bleached
-
Higher temperatures stress the polyps and lead to the expulsion of their mutualistic algae, causing the polyps to starve
-
Higher temperatures also disrupt the timing of mass spawning (a phenological event), potentially preventing polyps from reproducing
Ocean acidification also occurring as a consequence of increased carbon dioxide concentrations being absorbed by the ocean
-
Most of the carbon dioxide absorbed by the ocean combines with water to form carbonic acid (H2CO3)
-
The carbonic acid then dissociates into hydrogen carbonate ions and protons (H+), which lowers the pH of the water
Free carbonate ions will buffer this change by binding to the protons, but this reduces the availability of carbonate ions in the water
-
As these carbonate ions are typically used in the formation of calcium carbonate exoskeletons (in coral and shells), these living structures are damaged when ocean conditions become more acidic
Coral Bleaching
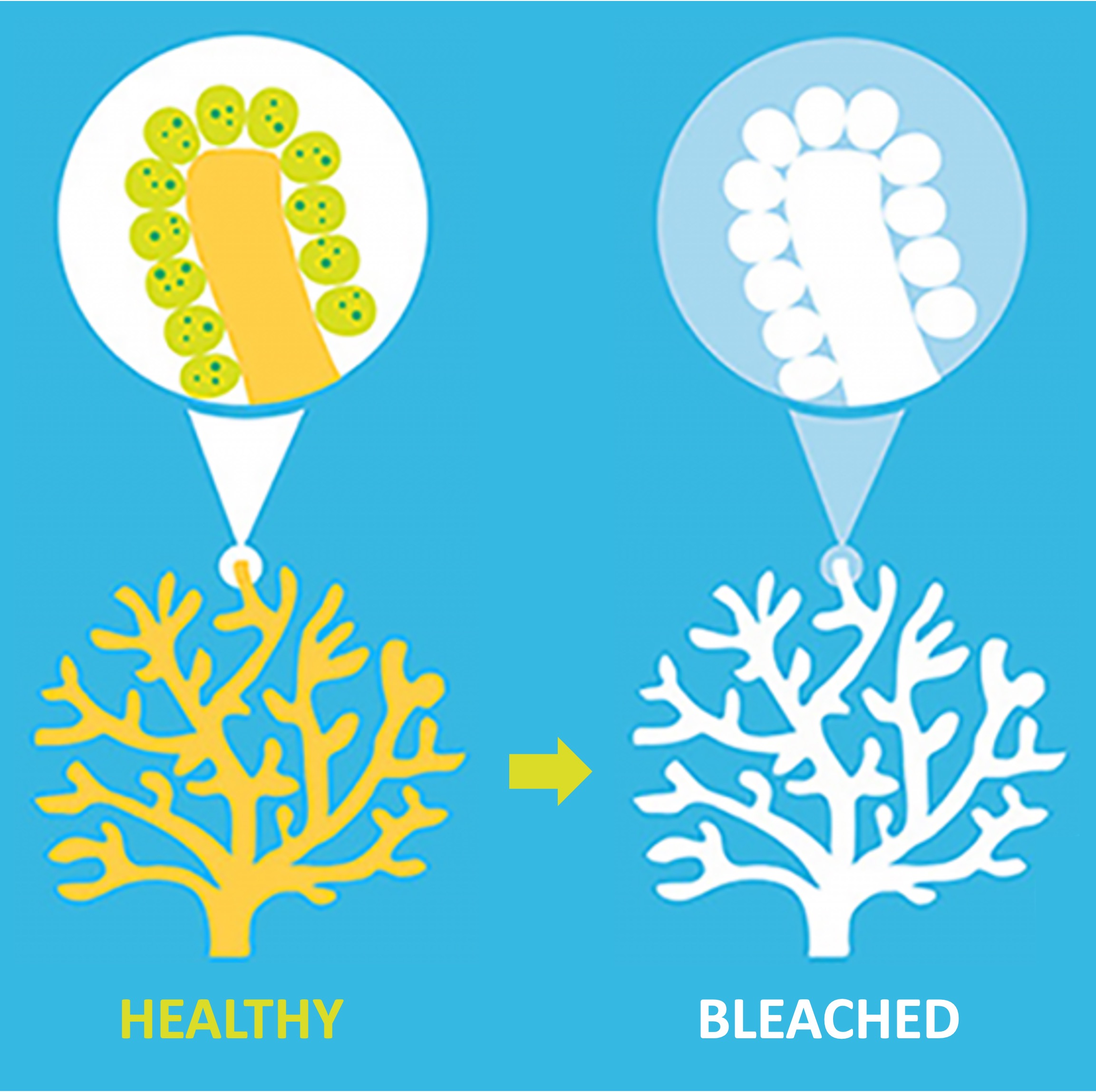
Algae Expulsion

Great Barrier Reef




