Lipid Functions
Energy Storage
Triglycerides in adipose tissues are used for long-term energy storage in animals
-
Triglycerides can store roughly twice as much energy per gram as carbohydrates and do not contribute to the osmotic pressure of the cell (as they are non-polar)
-
Triglycerides are more difficult to digest (they can only be broken aerobically) and cannot be easily transported due to their hydrophobicity
Consequently, triglycerides are more suitable for long-term energy storage whereas carbohydrates (glycogen) are more suitable for short-term energy storage



Thermal Insulation
Triglycerides have low thermal conductivity, meaning they have a limited capacity to conduct heat and are effective thermal insulators
-
Mammals living in cold or aquatic environments (such as the ringed seal) will possess thick layers of subcutaneous fat to insulate their internal organs against cold exposure
-
In humans, obese individuals tend to cool less rapidly, however the increased retention of heat makes them more susceptible to heat stress
Structure
Phospholipids are one of the key structural components of all cell membranes that are responsible for the formation of lipid bilayers
-
Phospholipids consist of a polar head (hydrophilic) composed of a glycerol and a phosphate molecule and two non-polar tails (hydrophobic) composed of fatty acid chains
-
Because phospholipids contain both hydrophilic (water-loving) and lipophilic (fat-loving) regions, they are classed as amphipathic
Phospholipids spontaneously arrange into a bilayer with the two hydrophobic tails being shielded from the surrounding polar fluids by the outward facing hydrophilic heads
-
The phospholipid bilayer is only held together by the weak hydrophobic associations between the non-polar tails, making the bilayer both fluid and flexible
-
Because phospholipids contain both hydrophilic (water-loving) and lipophilic (fat-loving) regions, they are classed as amphipathic
Phospholipid Properties


Communication
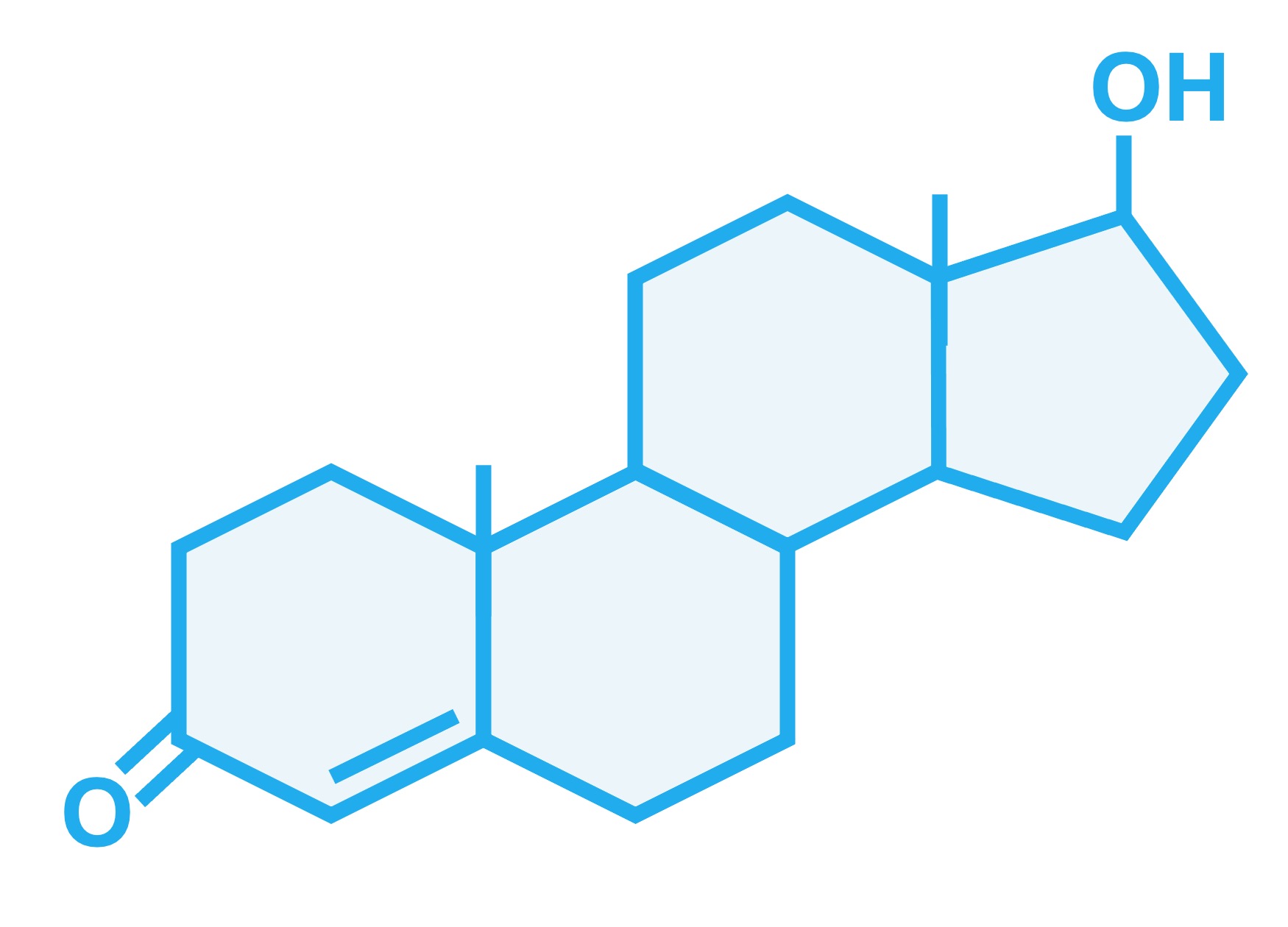
Steroids are lipids that are composed of four fused carbon rings that are non-polar and lipophilic (fat-loving)
-
Steroids do not resemble other types of lipids (they do not contain a fatty acid chain), but share their basic chemical properties (they are hydrophobic)
A steroid hormone is a steroid that functions as a signalling molecule within the body
-
Because they are lipophilic, they can freely diffuse across the phospholipid bilayer and bind to receptors within the target cell
-
However, because they are hydrophobic, they cannot be freely transported within the bloodstream and must be bound to carrier proteins (e.g. albumin)
Steroid hormones are generally synthesised from cholesterol in either the adrenal gland (corticosteroids) or the gonads (sex steroids)
-
Examples of sex steroids include oestradiol (a type of oestrogen) and testosterone
Steroid Hormones
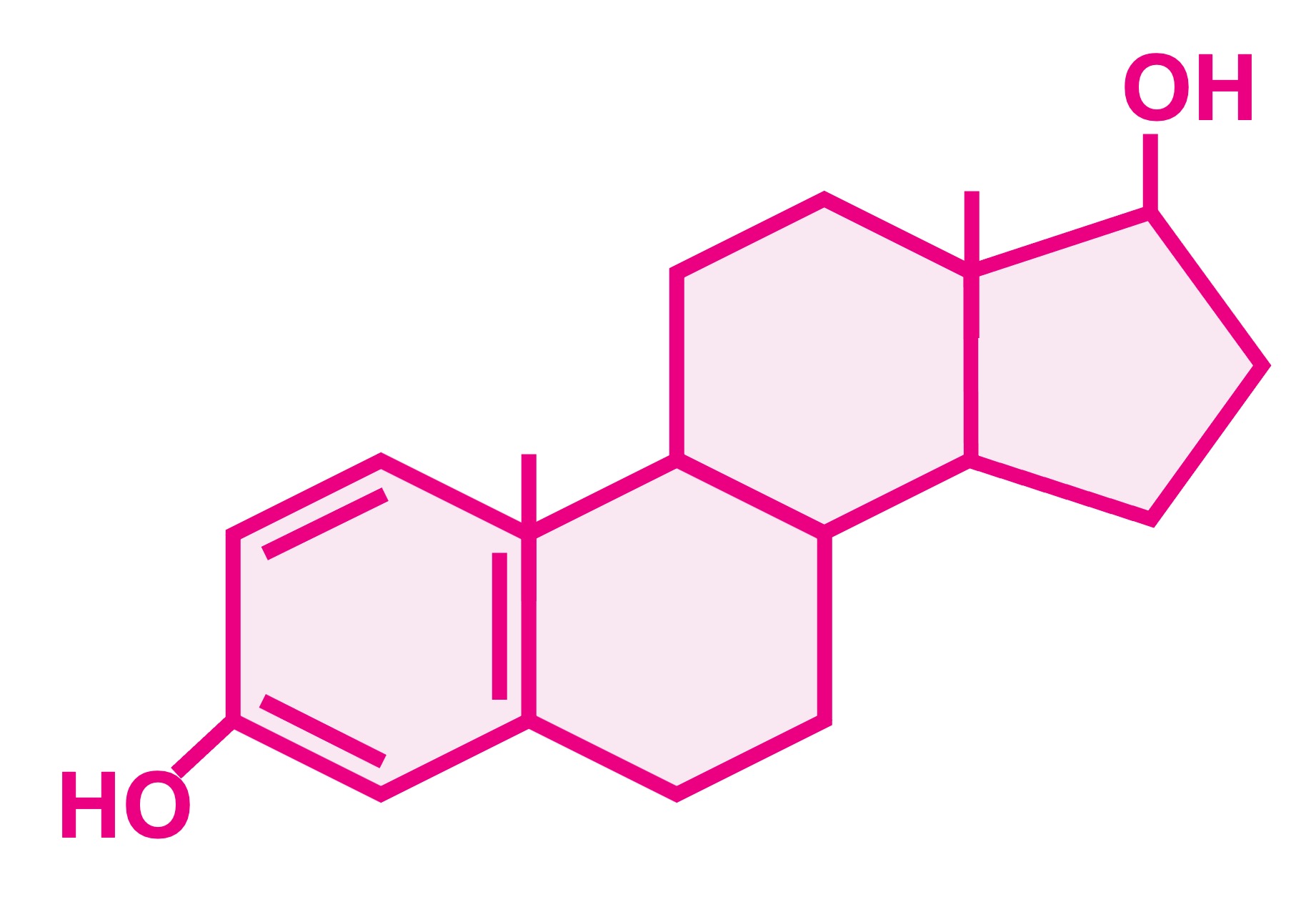
Oestradiol